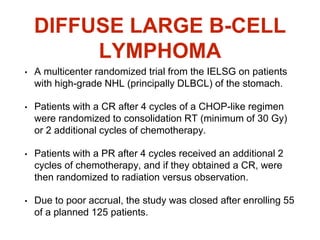
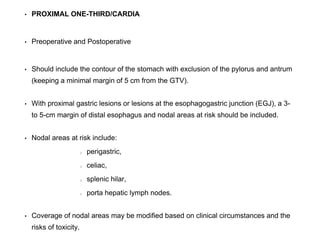
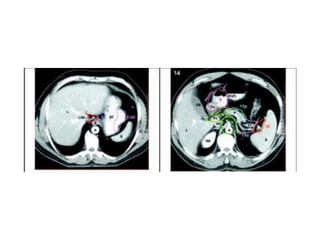
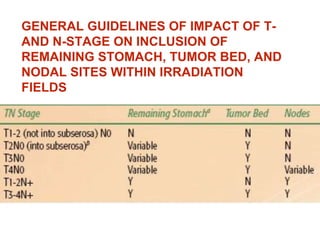
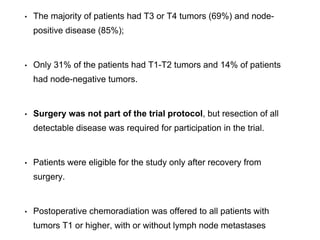
This document summarizes information about gastric carcinoma (stomach cancer). It covers the epidemiology, anatomy, pathology, risk factors, clinical presentation, staging, and treatment of gastric cancer. Key points include:
- Gastric cancer was previously a leading cause of cancer death but now ranks fourth most common. Incidence is highest in China and Japan.
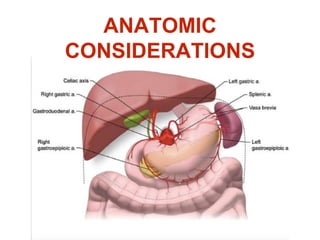
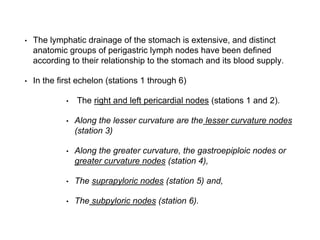
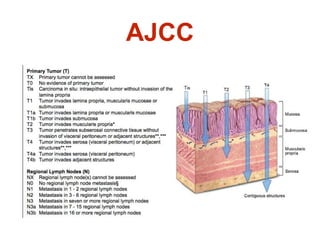
- The stomach has extensive lymphatic drainage involving 16 lymph node stations.
- 95% of gastric cancers are adenocarcinomas. Other rare types include squamous cell carcinoma and carcinoid tumors.
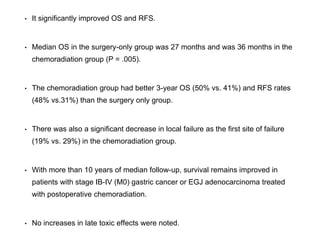
- Risk factors include smoking, obesity, and H. pylori infection. Symptoms are often vague but may include weight loss, abdominal pain,











![POSITRON EMISSION
TOMOGRAPHY
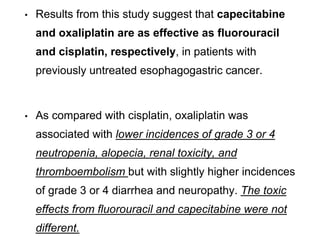
• PET/CT was tested as a tool to predict response to neoadjuvant
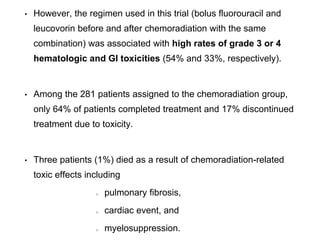
chemotherapy.
• Ott et al., reported 90% 2-year survival in patients with PET-defined
response <35% decrease standardized uptake value
[SUV]) versus
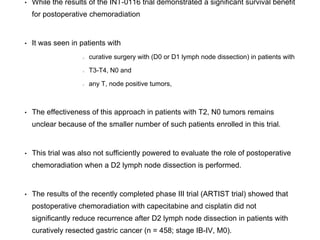
25% for patients not responding to PET.
• PET response could be detected as early as 14 days.
• The role of PET/CT in the primary staging of gastric cancer
remains to be established.](https://image.slidesharecdn.com/gastricca-170407093255/85/Gastric-carcinoma-19-320.jpg)





















![• In the early 1980s, FAM (fluorouracil, doxorubicin, and
mitomycin) was considered the gold standard for patients
with advanced gastric cancer.
• ECF demonstrated improvements in median survival and
quality of life
• In 2006, the FDA approved the DCF regimen for the
treatment of patients with advanced gastric cancer,
including EGJ cancers, in patients who have not received
prior chemotherapy [overall response rate (ORR) of 37%
with DCF and 25% with CF, (P = .01)].](https://image.slidesharecdn.com/gastricca-170407093255/85/Gastric-carcinoma-57-320.jpg)
![• The REAL-2 (with 30% of patients having an esophageal
cancer) trial was a randomized multicenter phase III
study comparing capecitabine with fluorouracil and
oxaliplatin with cisplatin in 1003 patients with advanced
esophagogastric cancer.
• ARMS-
epirubicin, cisplatin, fluorouracil[ECF];
epirubicin, oxaliplatin, fluorouracil [EOF];
epirubicin, cisplatin, and capecitabine [ECX]; and
epirubicin, oxaliplatin, and capecitabine [EOX]).](https://image.slidesharecdn.com/gastricca-170407093255/85/Gastric-carcinoma-58-320.jpg)