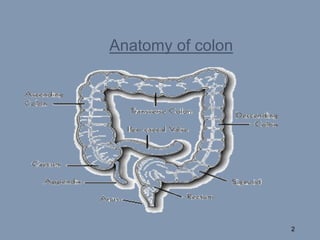
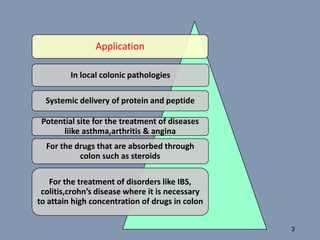
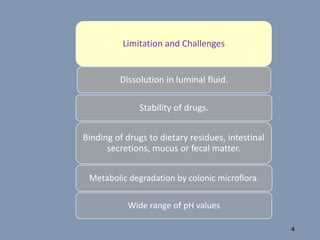
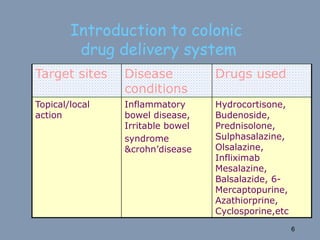
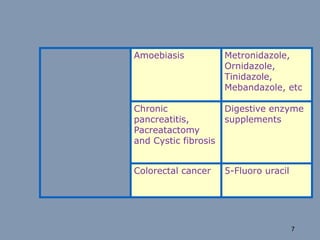
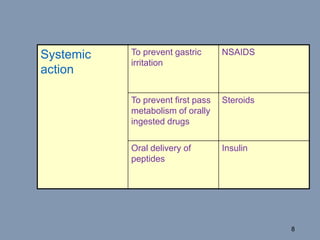
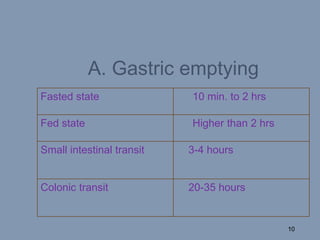
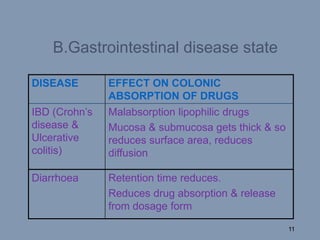
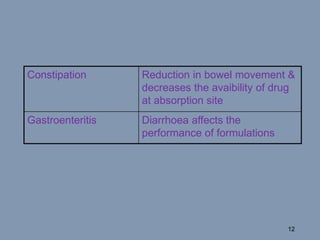
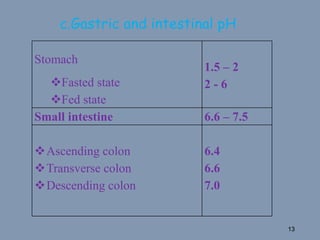
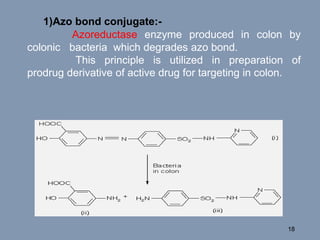
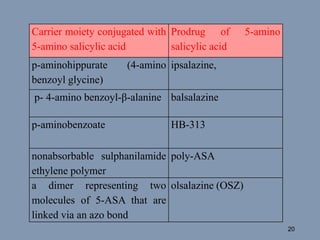
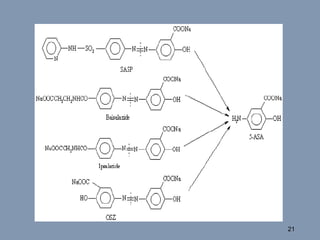
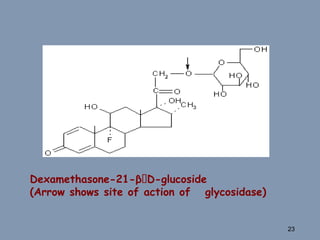
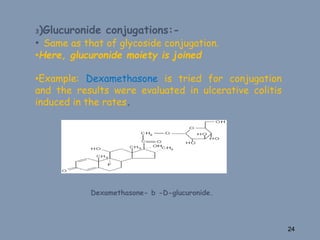
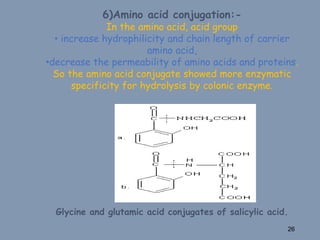
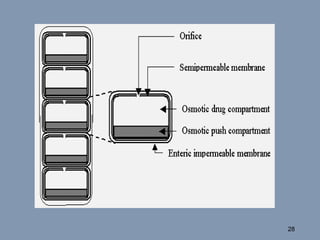
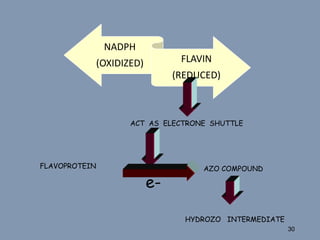
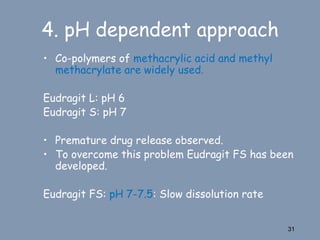
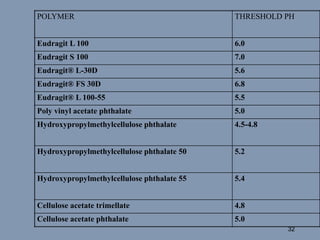
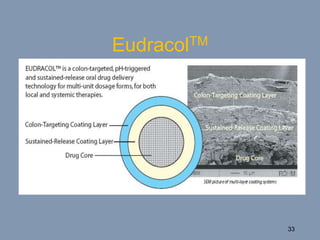
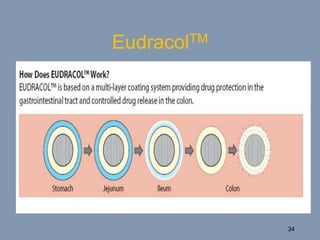
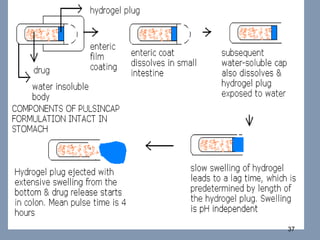
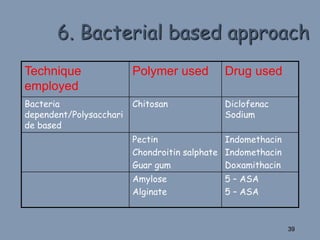
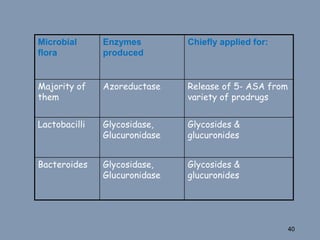
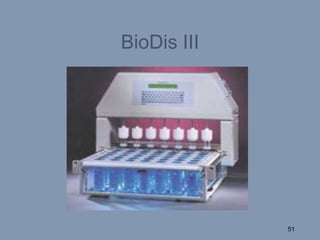
This document discusses colon-targeted drug delivery systems, outlining their applications in treating local colonic pathologies and systemic diseases, and detailing various methods and challenges related to drug transport in the colon. It highlights different approaches for managing drug stability and absorption, including prodrug strategies, osmotic delivery, and bioadhesive systems, while also addressing factors that affect drug delivery in certain gastrointestinal conditions. The document emphasizes the significance of innovative formulations and methods to improve drug release profiles and therapeutic efficacy in the colon.