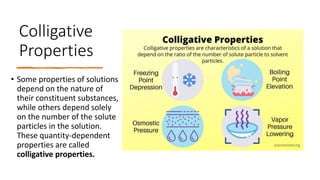
Colligative properties depend only on the number of solute particles and not their identity. They include lowering of vapor pressure, freezing point depression, boiling point elevation, and osmotic pressure. Electrolytes have a greater effect on colligative properties than non-electrolytes because they dissociate into ions. Raoult's law states that vapor pressure of a solution is directly proportional to mole fraction of the solvent and inversely proportional to mole fraction of the solute.