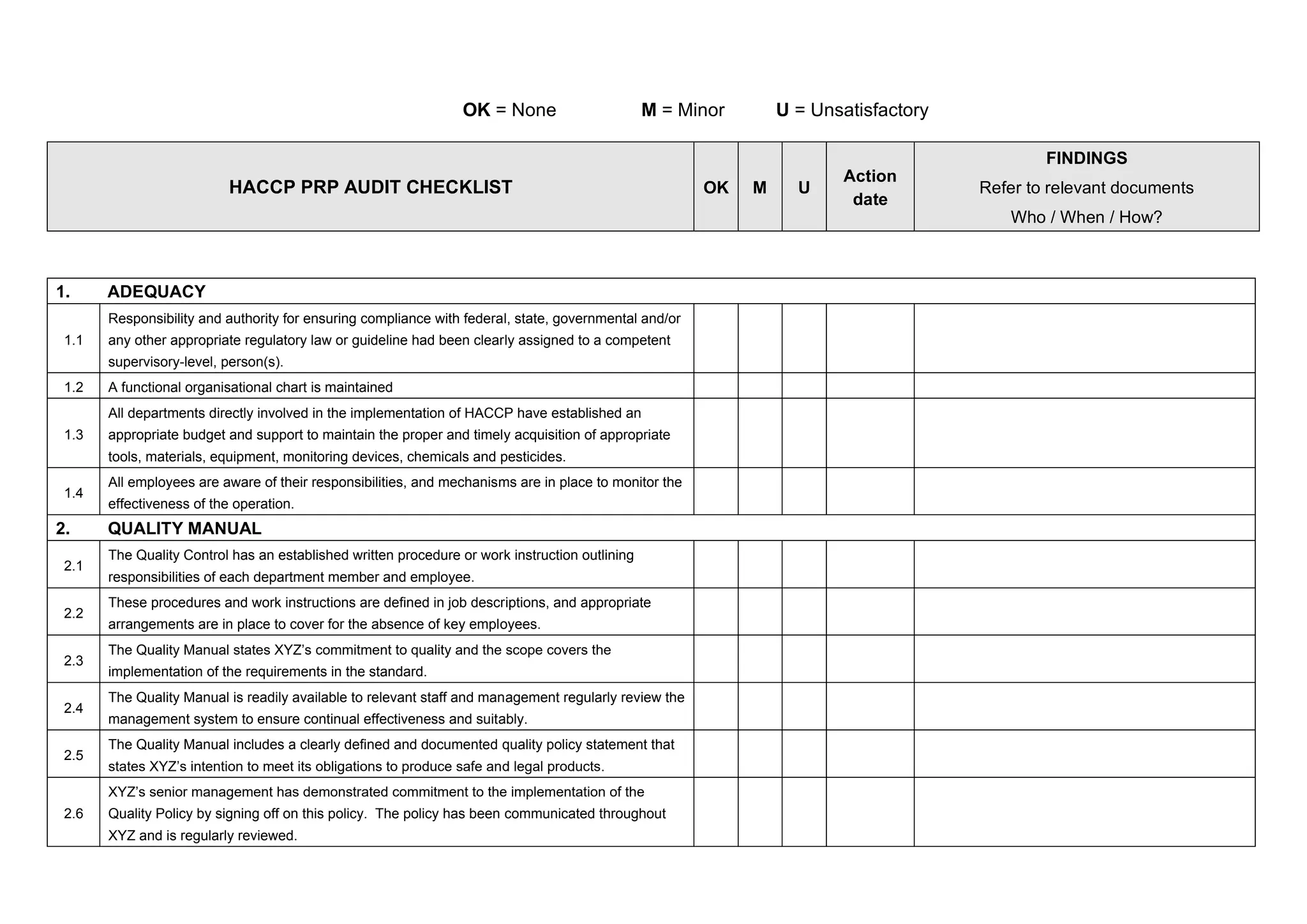
This document contains an audit checklist evaluating compliance with Hazard Analysis and Critical Control Point (HACCP) programs and procedures. It assesses areas like responsibility and authority, quality manuals, internal audits, cleaning procedures, documentation of incoming materials, the HACCP program, training, product recalls, and procedures for non-conforming products. Compliance is rated as OK, Minor issue, or Unsatisfactory. Findings and corrective actions are documented with references to relevant documents, responsible parties, and deadlines.