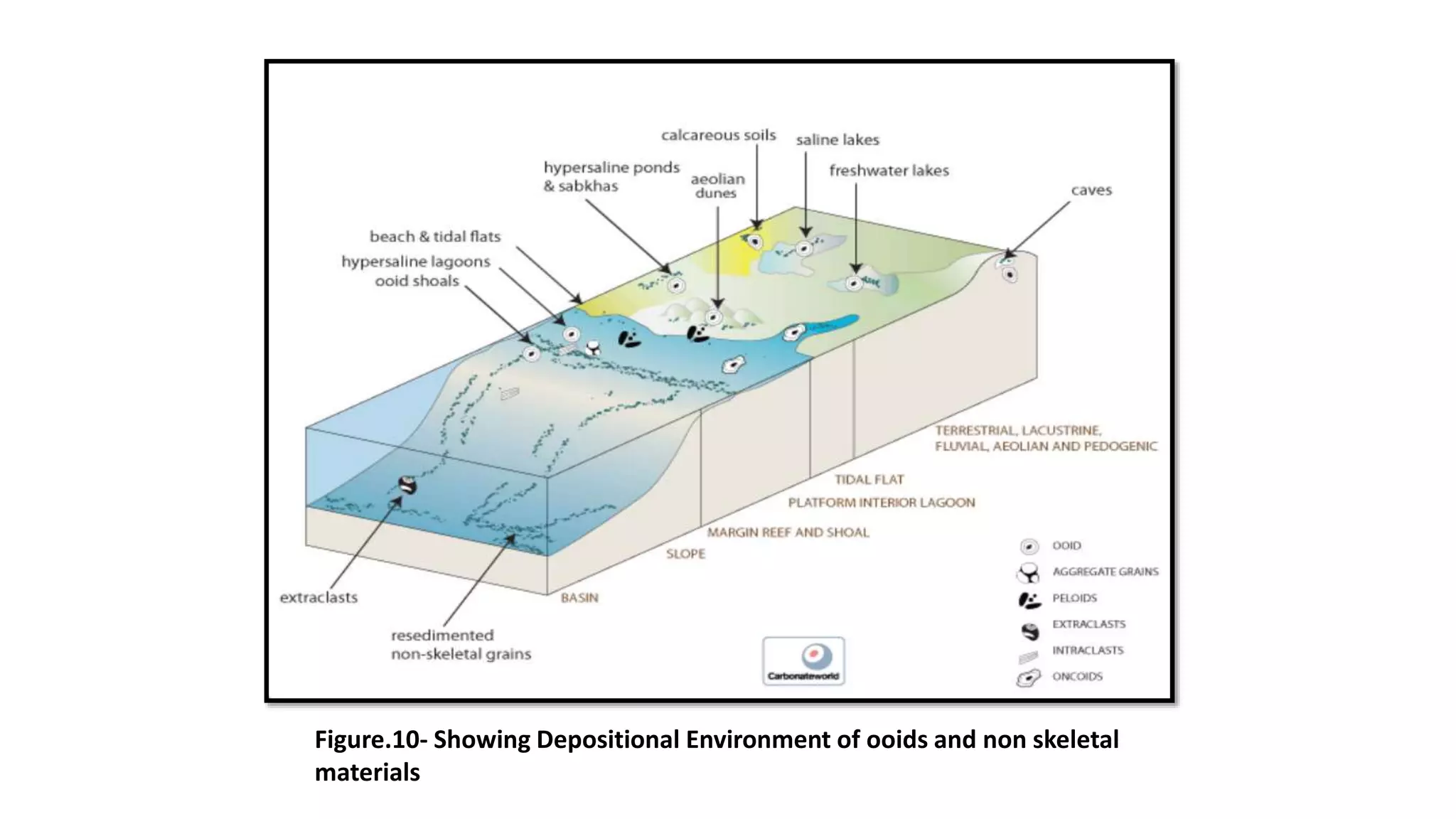
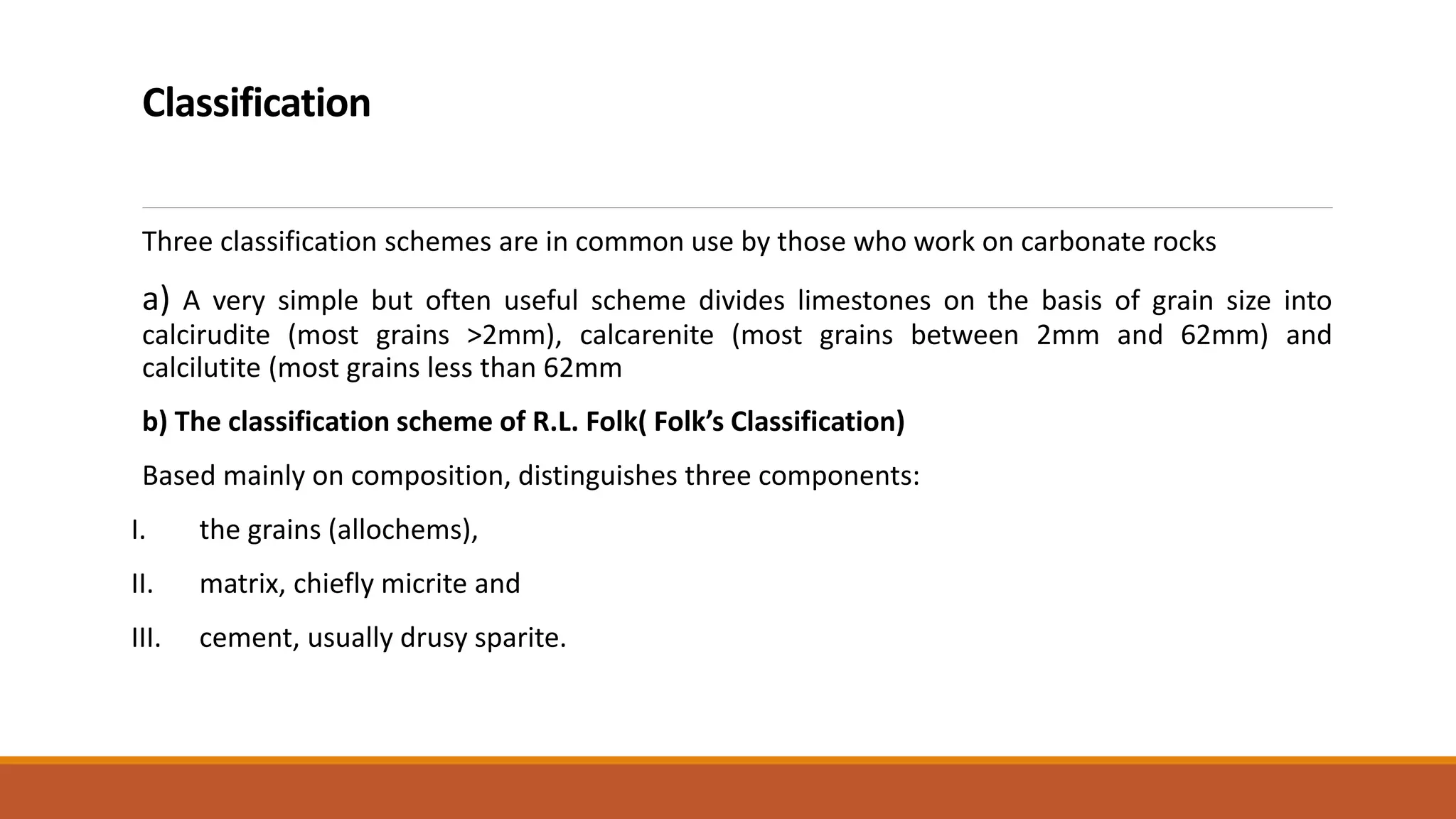
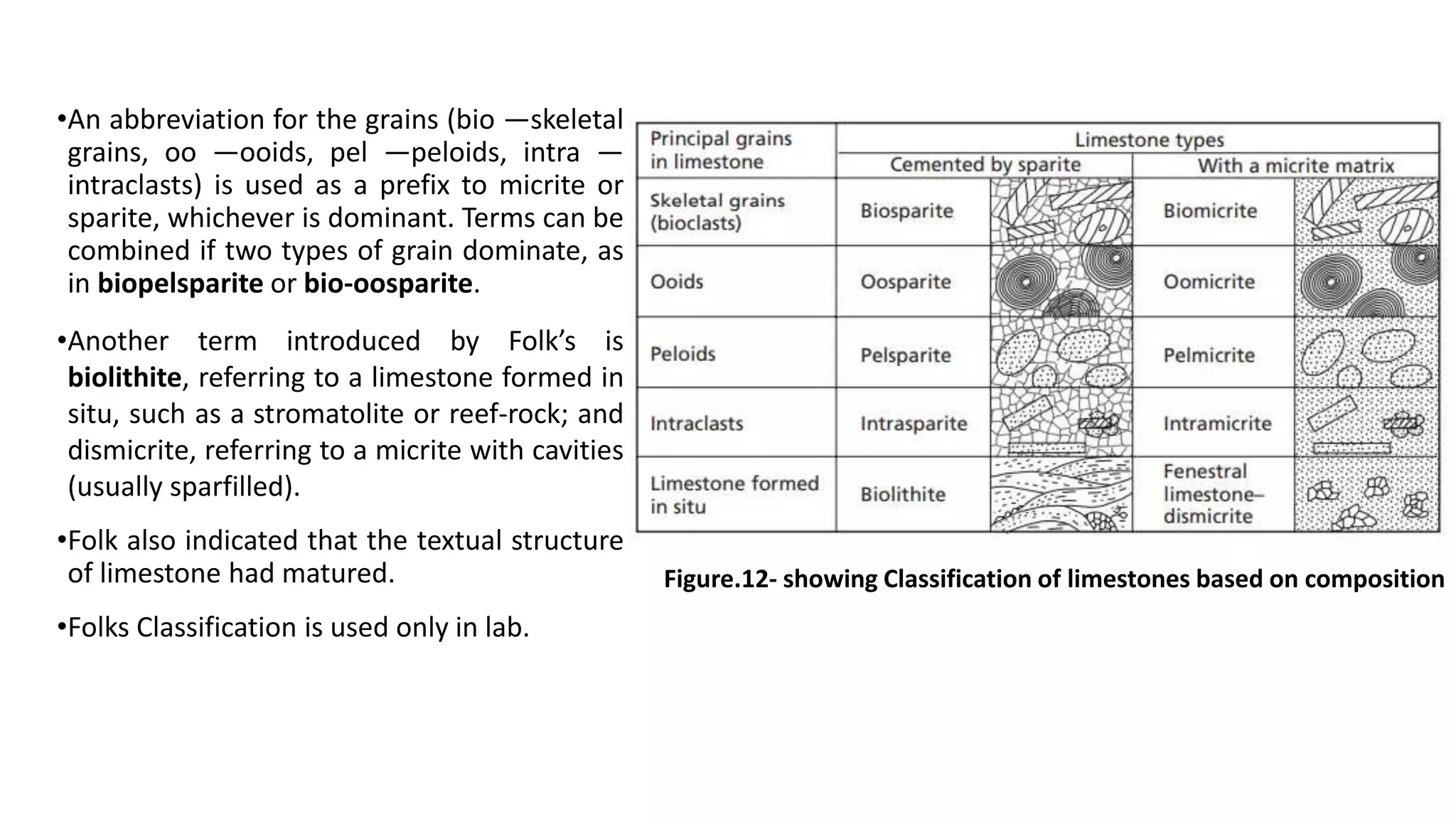
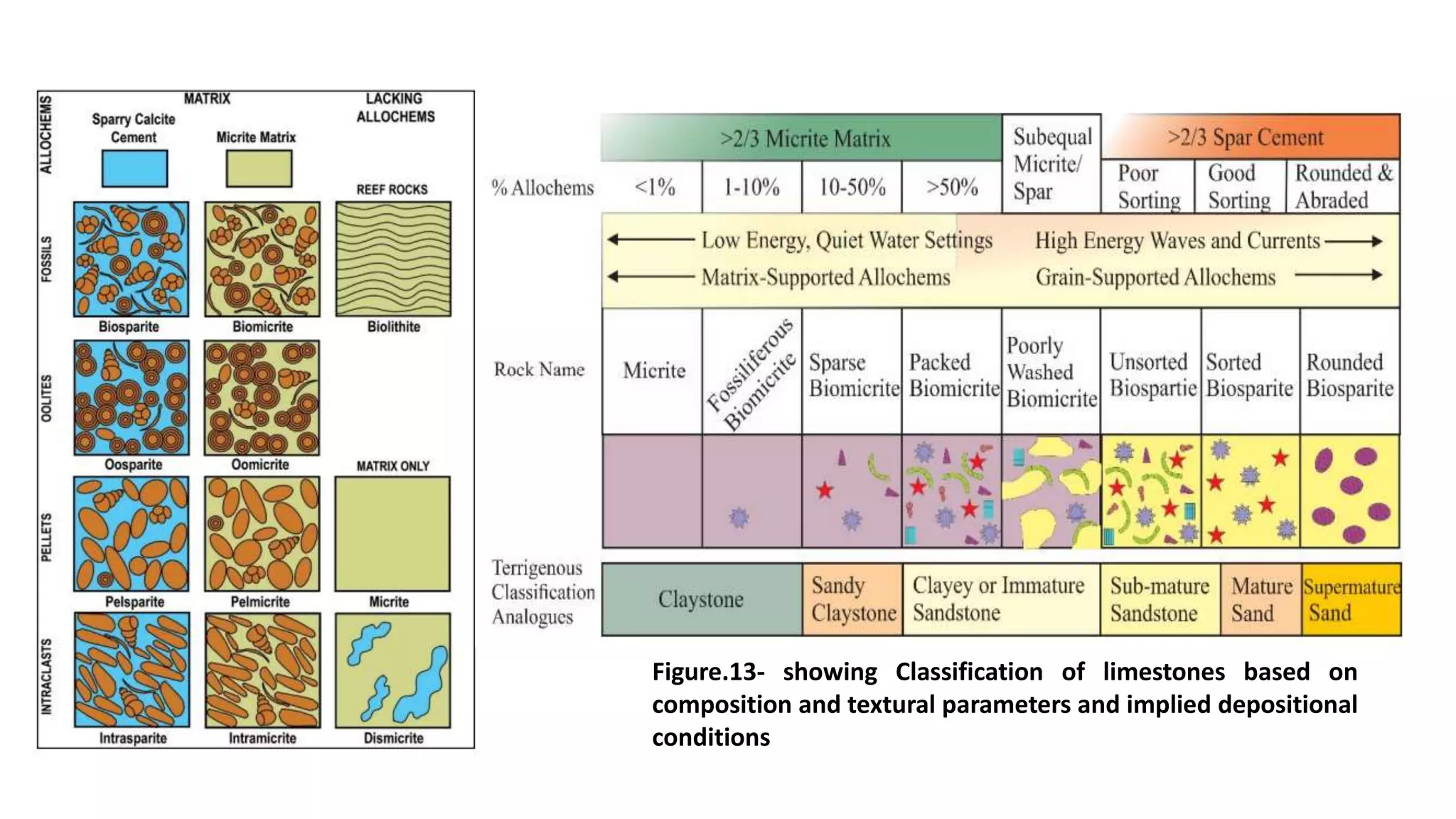
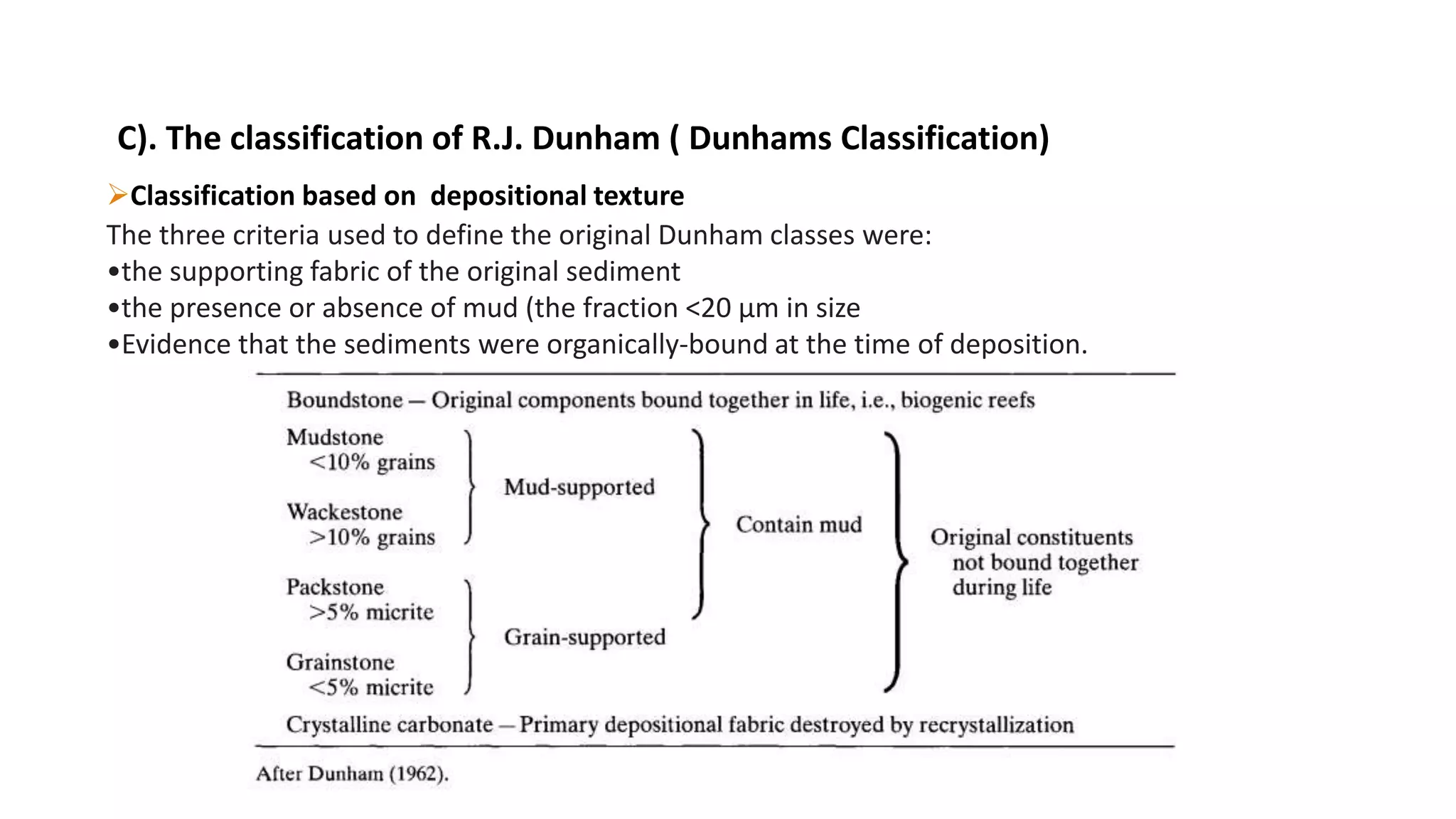
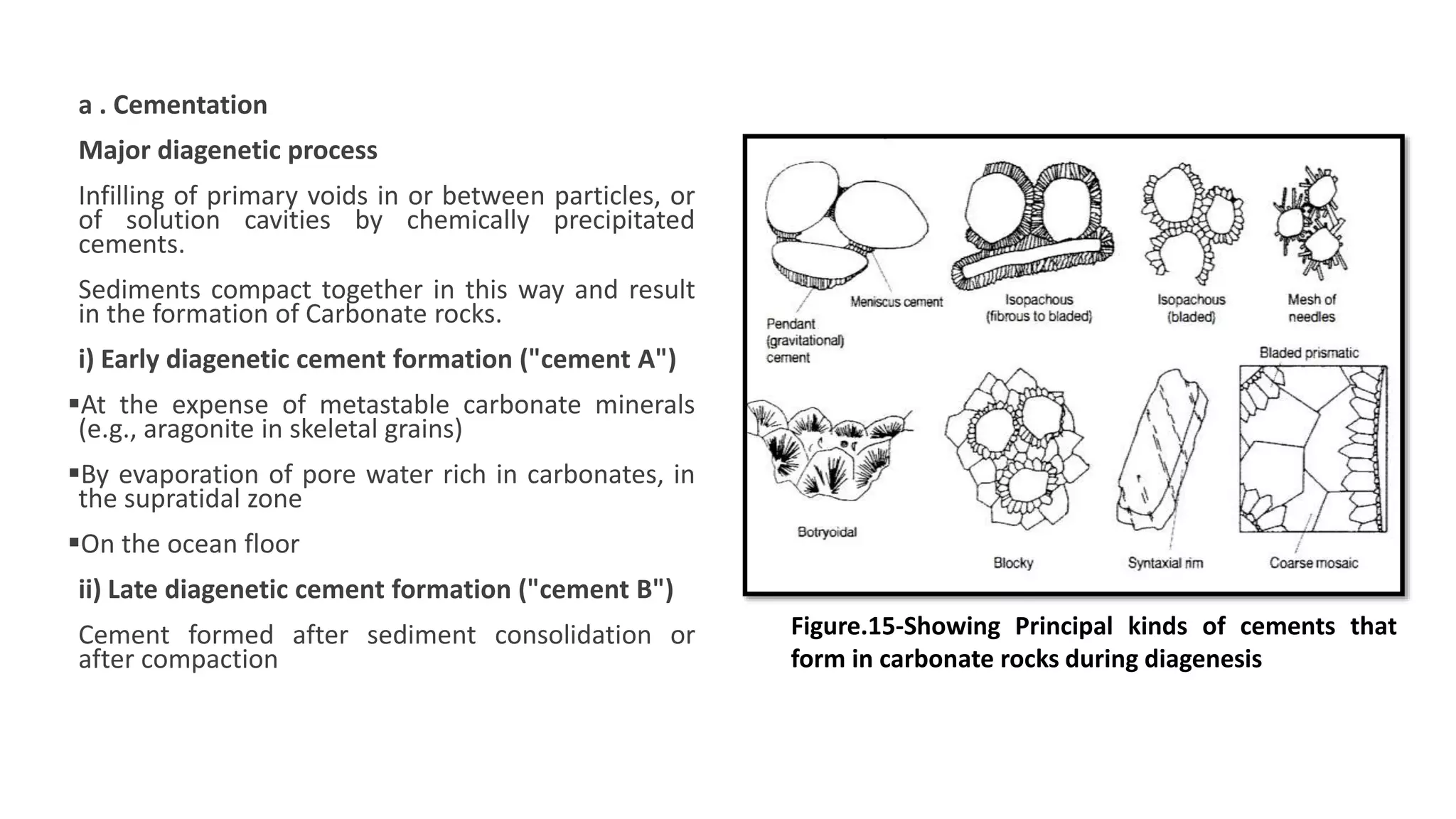
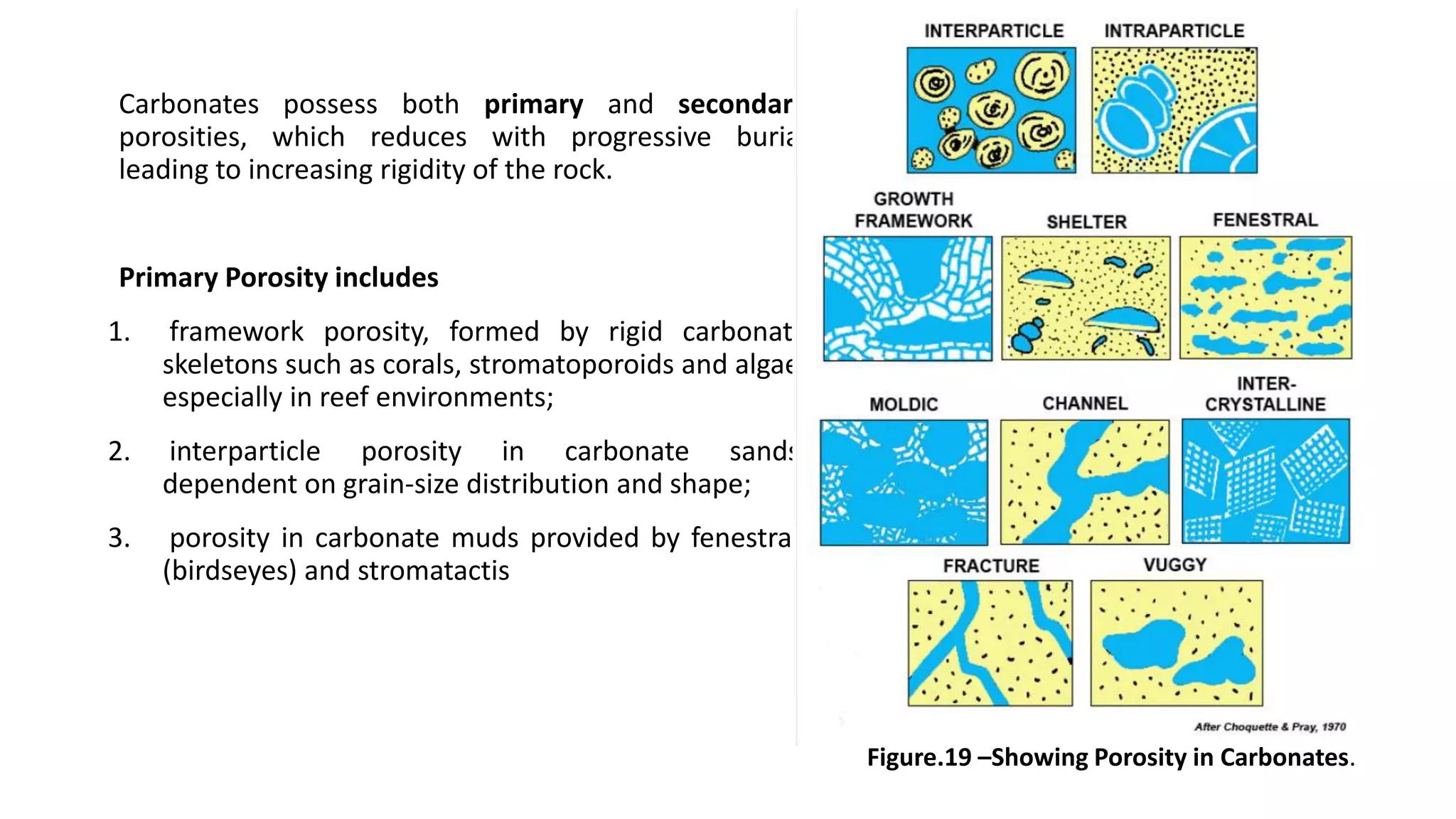
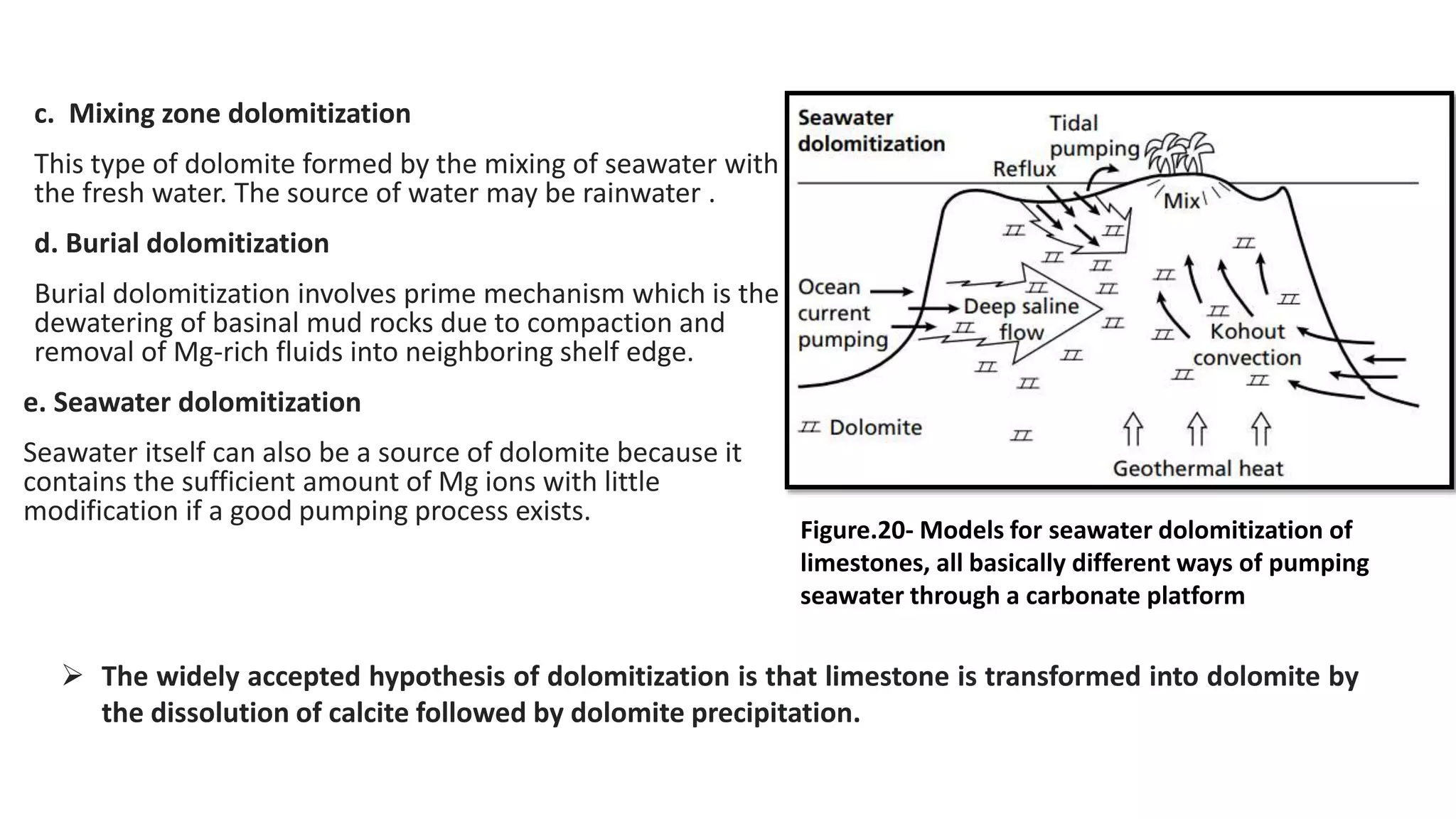
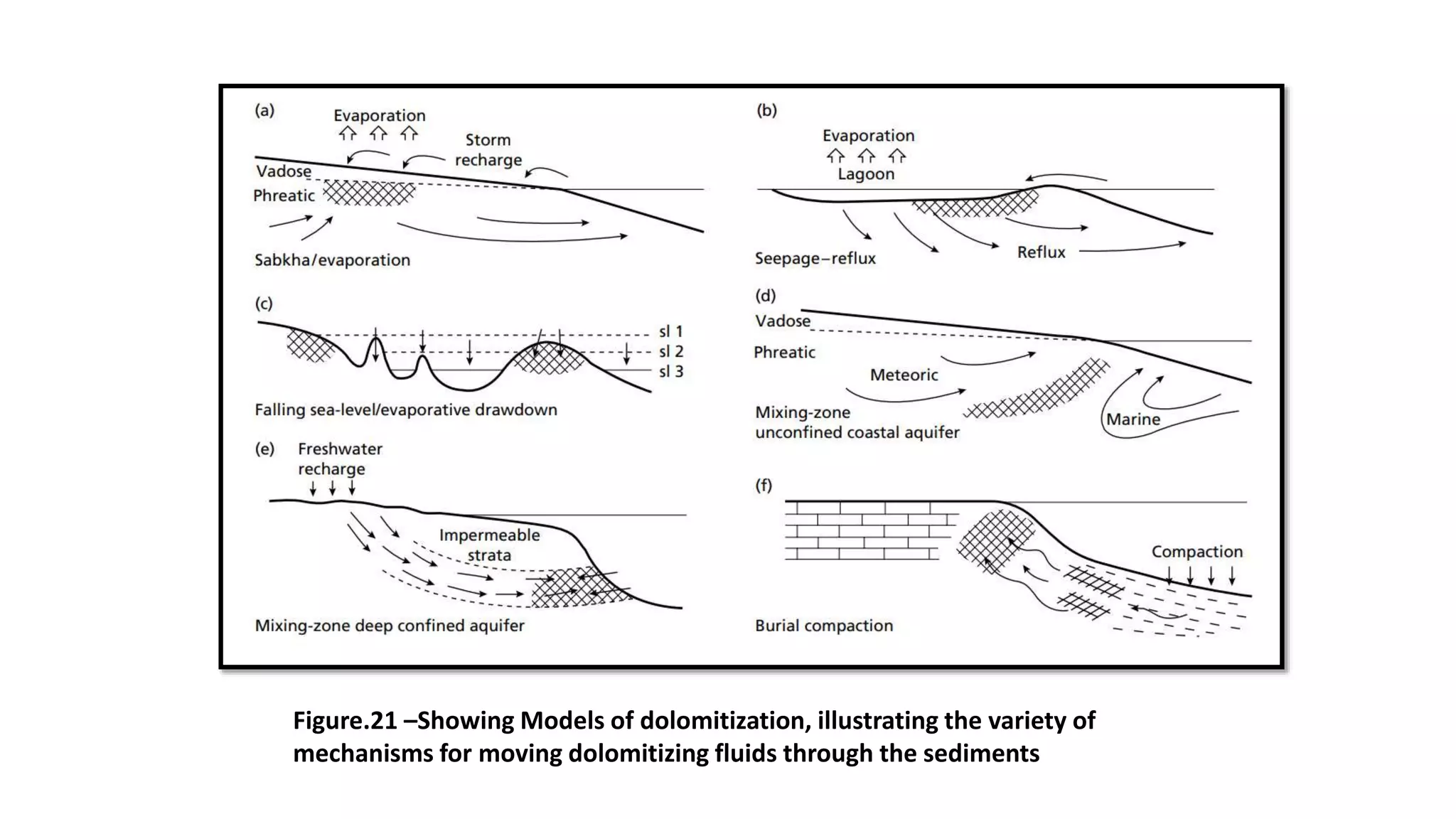
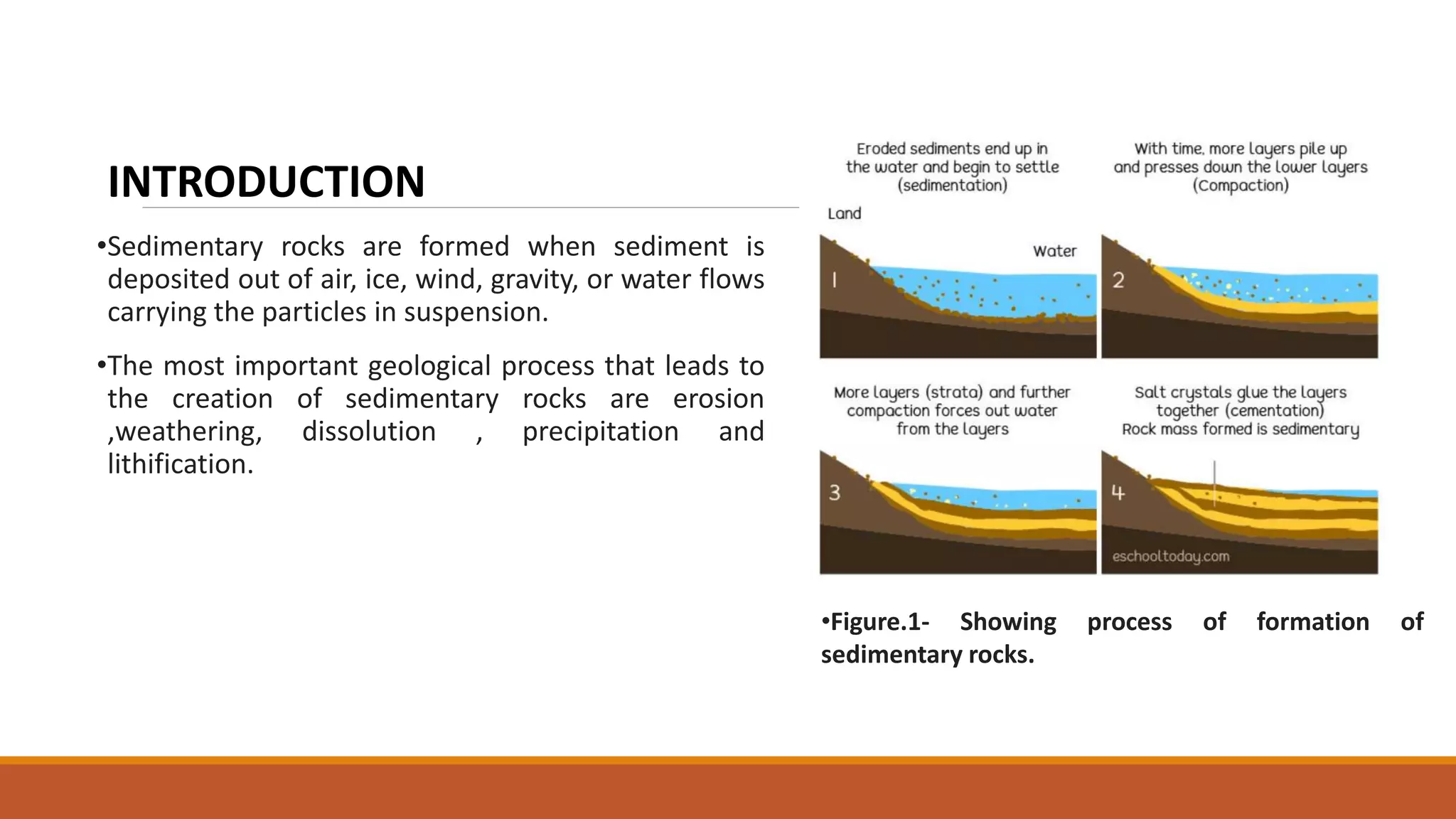
Carbonate sedimentary rocks form from the precipitation of minerals from water through chemical or biochemical processes. They include limestones composed of calcite and dolostones composed of dolomite. Carbonate rocks are classified based on their components, including allochems like skeletal grains and non-skeletal grains, and orthochems like micrite and spar. Robert Dunham's classification system categorizes carbonate rocks based on depositional texture as mudstones, wackestones, packstones, or grainstones, indicating the energy of the depositional environment.



![CON’T
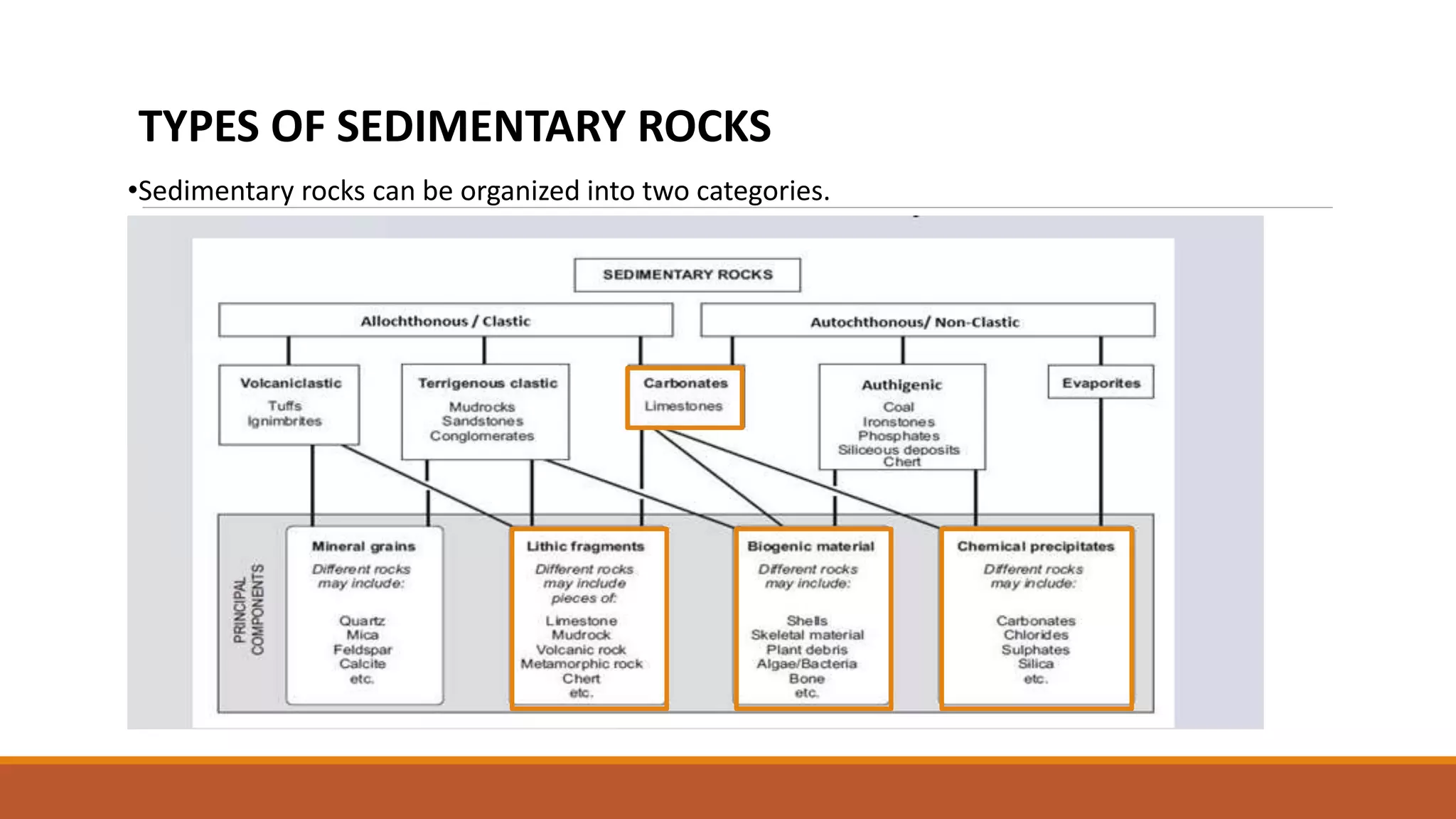
•The carbonate rocks make up 10 to 15% of sedimentary rocks. They largely consist of two types
of rocks and these two are most abundant Carbonate rocks.
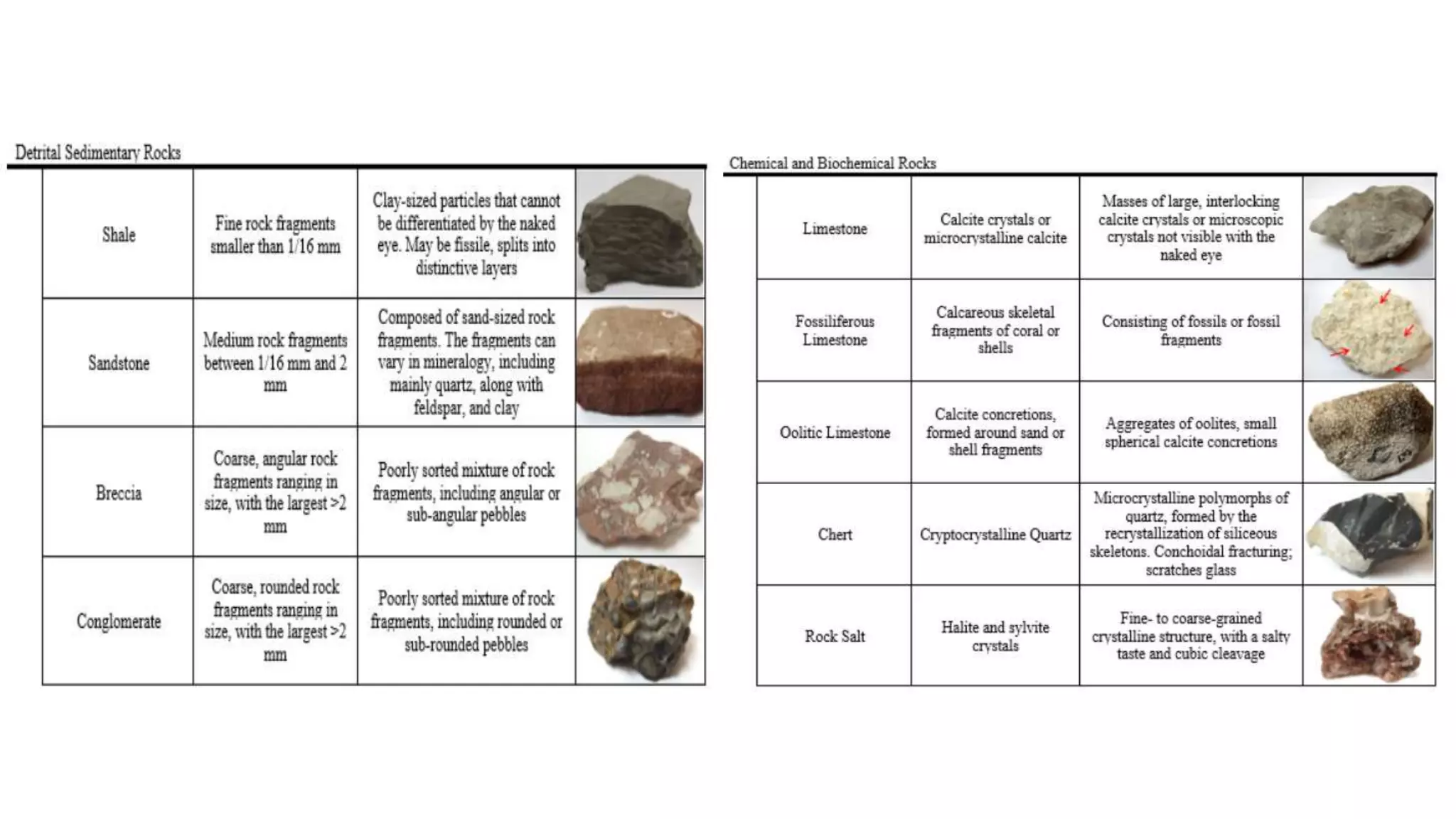
a) Limestones which are composed mostly of calcite (CaCO3) or high Mg calcite [(Ca,Mg)CO3],
and
b) Dolostones which are composed mostly of dolomite [CaMg(CO3)2]
•They are present in many Precambrian assemblages and in all geologic systems from the
Cambrian to the Quaternary.
a. Precambrian-Paleozoic - Dolomite
b. Mesozoic and Cenozoic Carbonates –Limestone
•Another common carbonate rock, containing a mixture of fine-grained calcite and terrigenous
mud, is marl.](https://image.slidesharecdn.com/classification-of-sedimentary-rocks-autochthonous-sediments-230818141217-0c52db88/75/Classification-of-sedimentary-rocks-Autochthonous-sediments-pptx-8-2048.jpg)