This document provides information about atoms, molecules, ions and chemical formulas. It discusses key concepts such as:
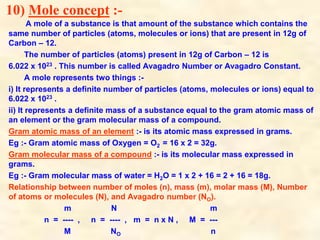
- Dalton's atomic theory which states that matter is made of tiny indivisible particles called atoms that combine in small whole number ratios.
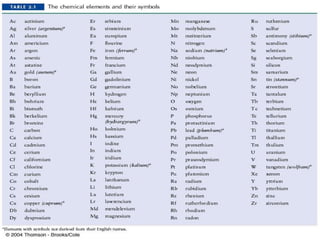
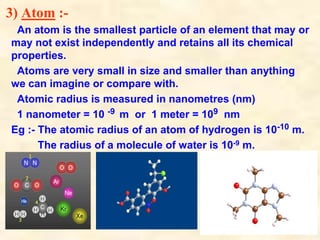
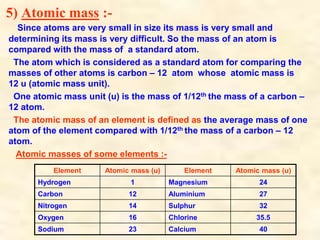
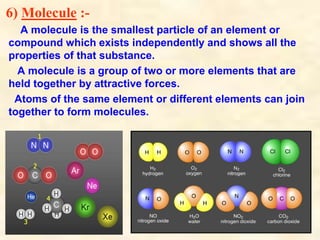
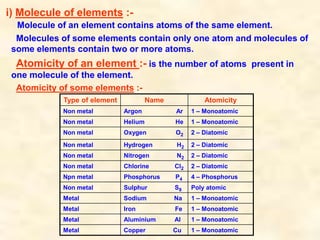
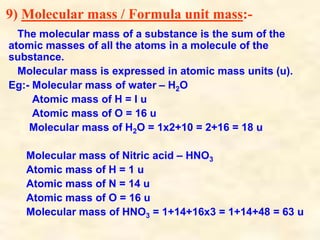
- Atoms have symbols to represent them and an atomic mass that is measured relative to carbon-12. Molecules are groups of atoms that are chemically bonded.
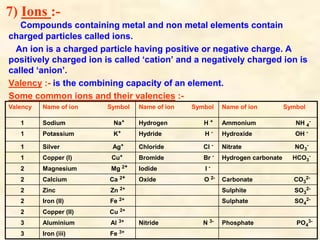
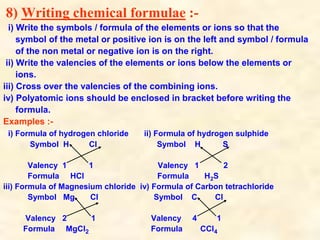
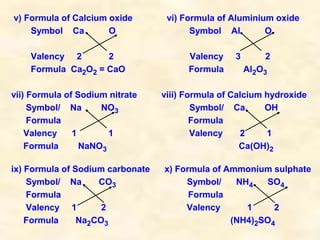
- Chemical formulas show the types and numbers of atoms or ions that make up a compound. Formulas are written with the cation written first followed by the anion.