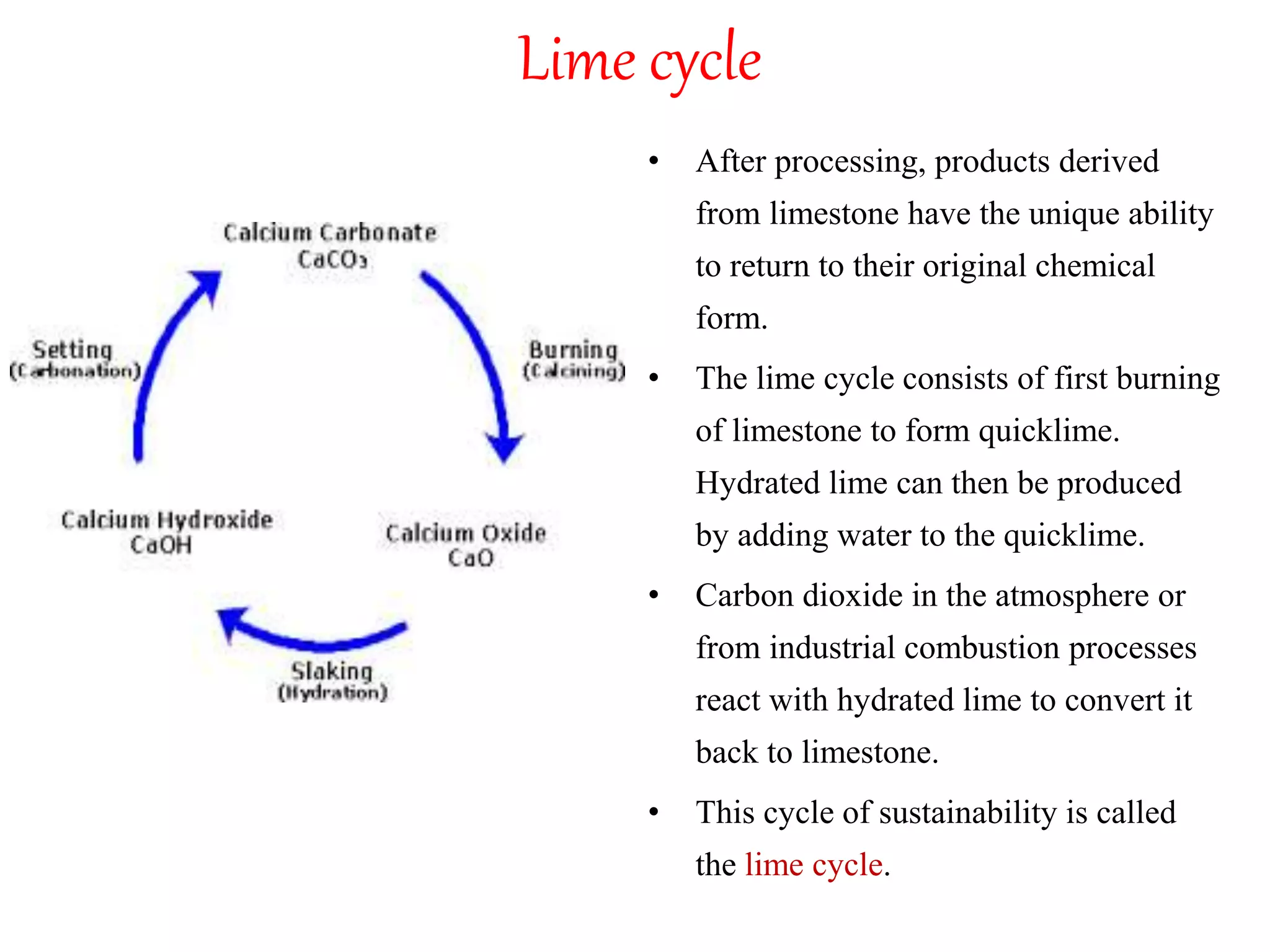
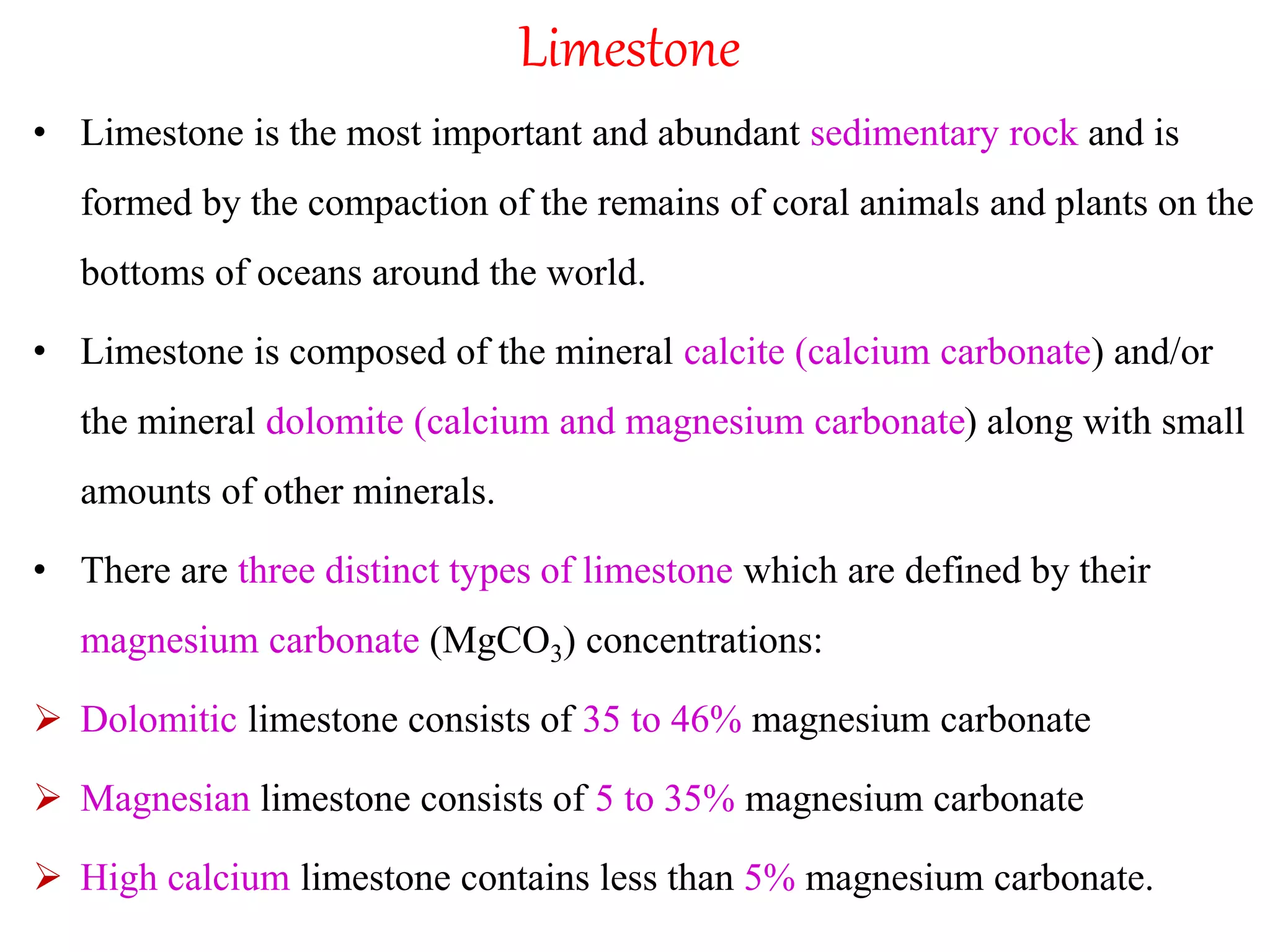
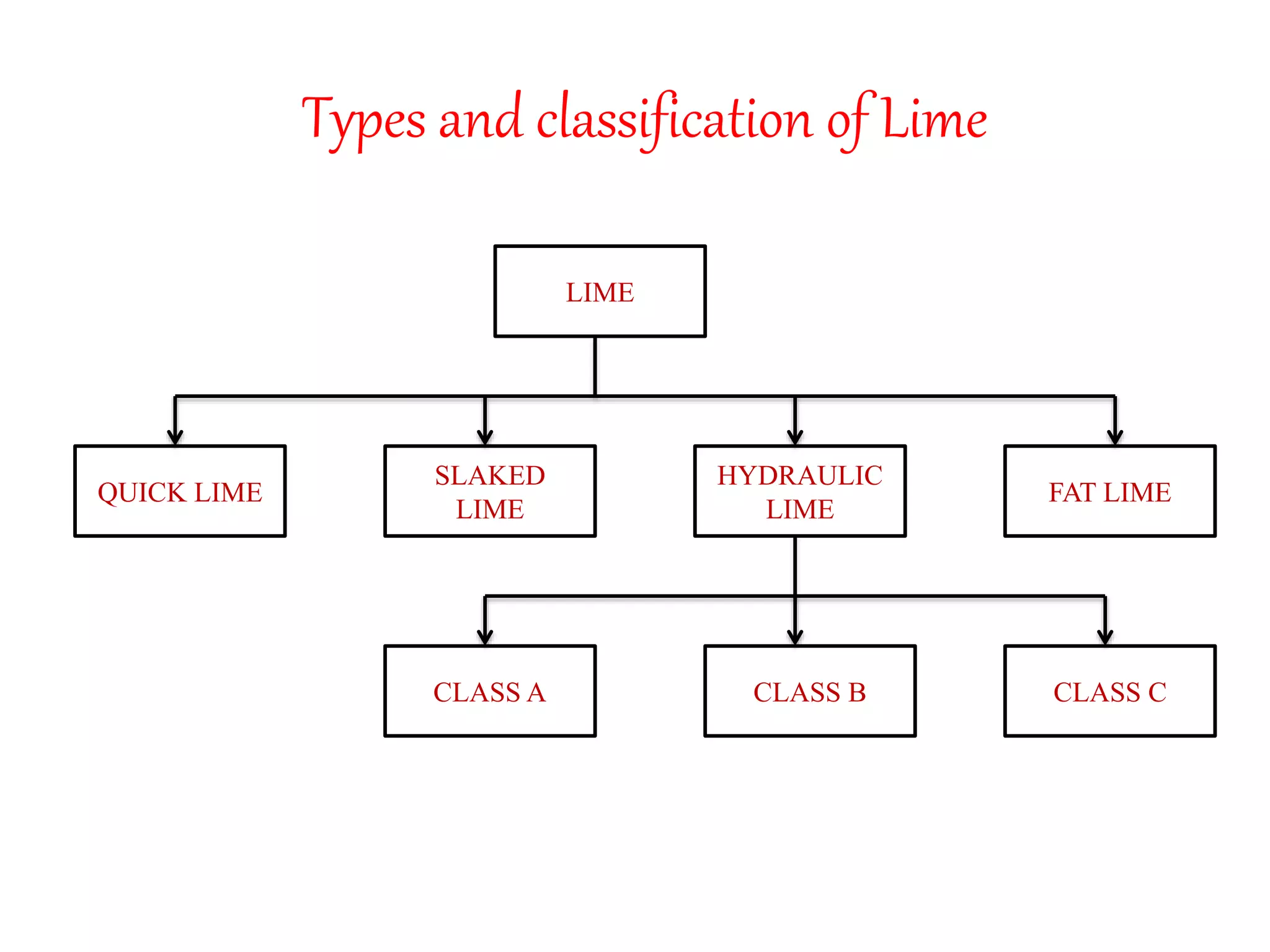
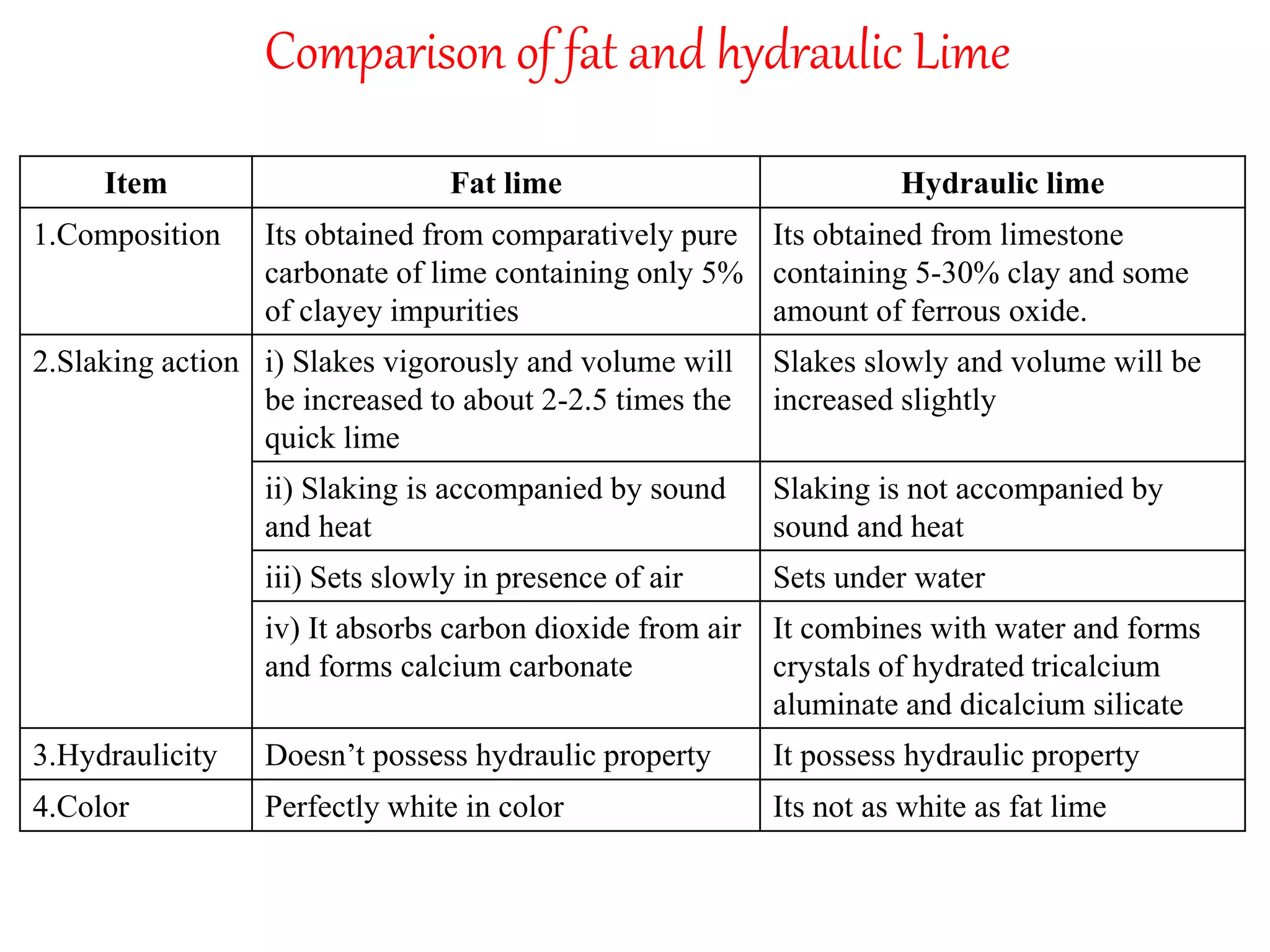
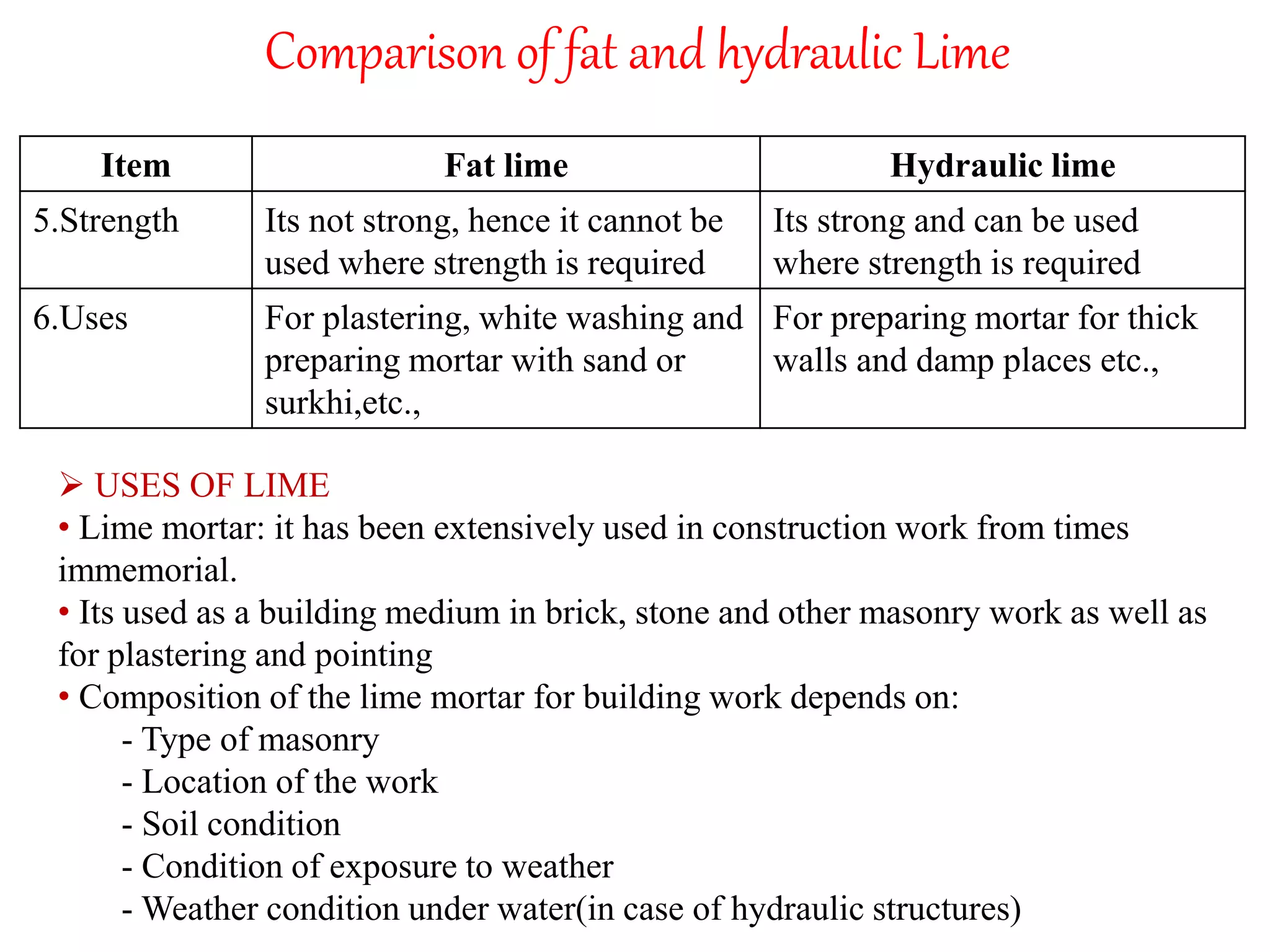
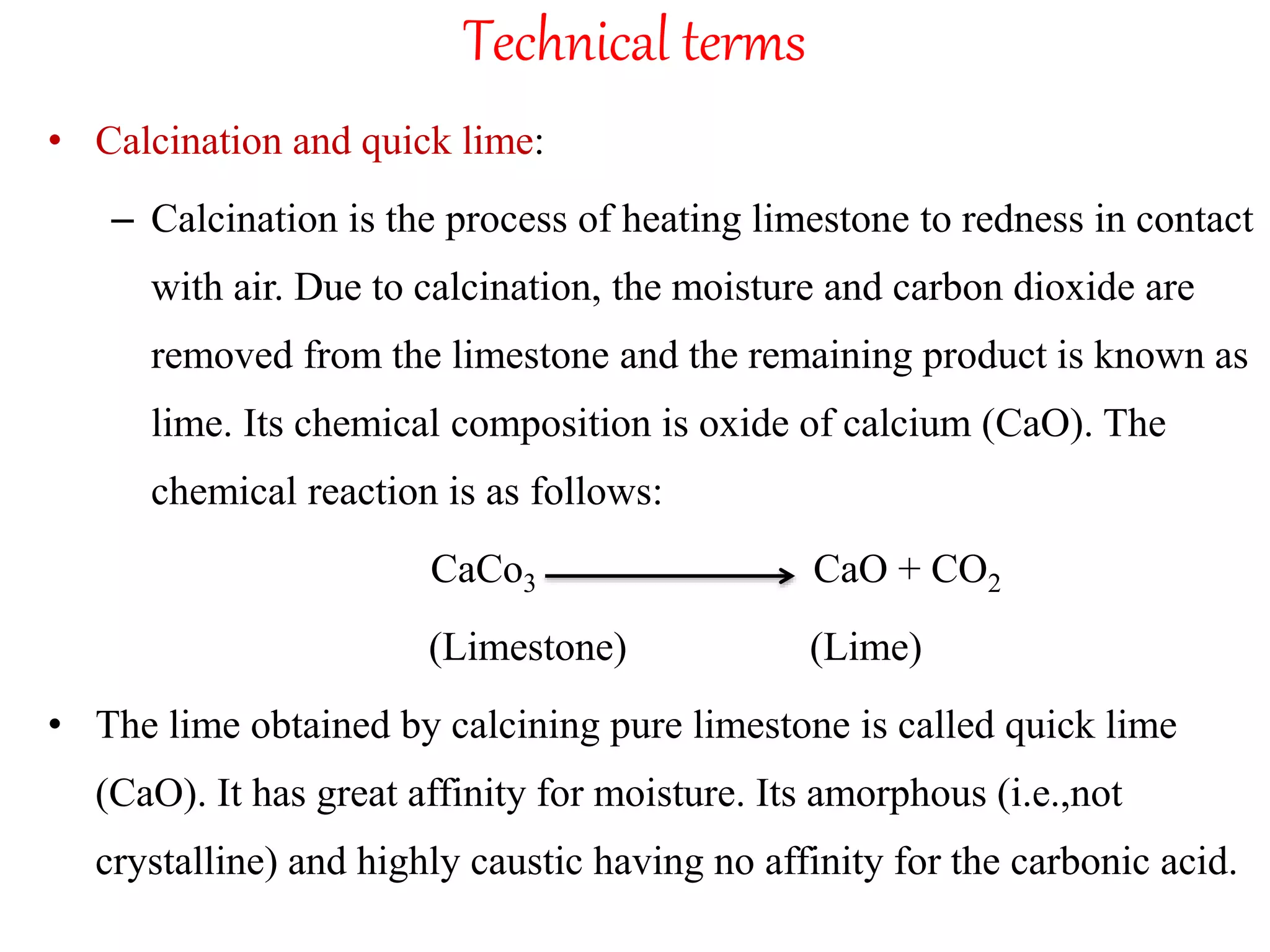
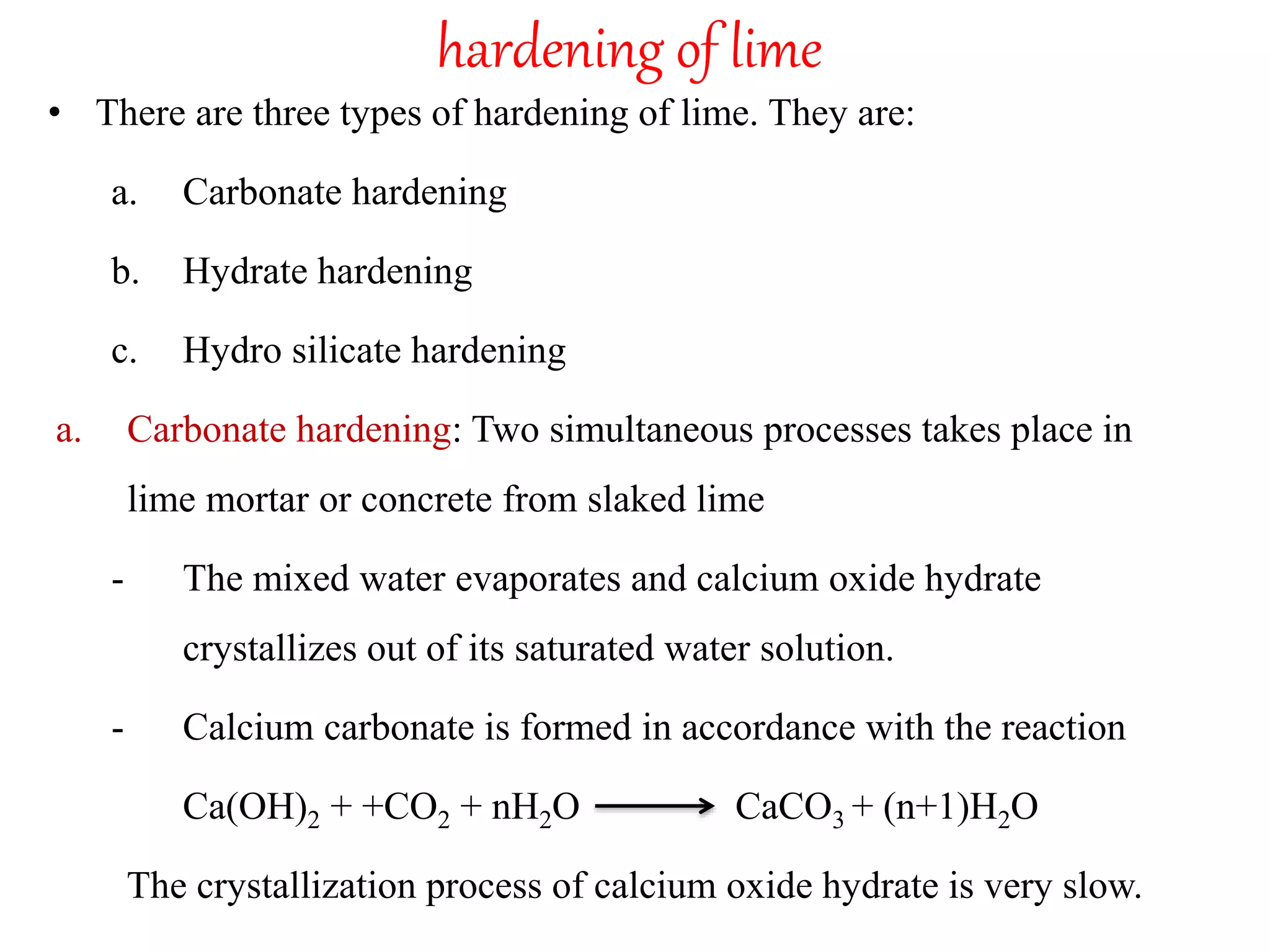
The document discusses lime, including its types, classification, manufacturing process, properties, and uses. It defines lime as products derived from burnt limestone such as quicklime and hydrated lime. There are three main types - quick lime, slaked lime, and hydraulic lime. Lime is classified based on its hydraulic properties into Class A, B, and C. The manufacturing process involves collecting limestone, burning it in kilns or clamps to produce quicklime, and then slaking the quicklime with water. Lime is used widely in construction for mortar, plaster, and concrete due to its binding properties.