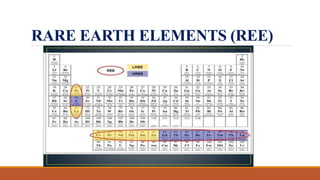
This document discusses trace elements in geology. It defines trace elements as chemical elements that exist in very small concentrations in rocks and minerals, generally less than 0.1% by weight. Trace elements are divided into incompatible and compatible elements based on how readily they substitute for major elements in minerals. Rare earth elements, a group of 15 elements from lanthanum to lutetium, are also discussed. The document outlines several ways that trace elements are important for understanding geological processes, mineral formation, and environmental conditions.