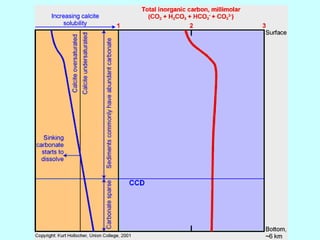
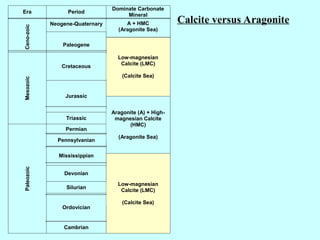
Chapter 6 discusses carbonate sedimentary rocks, primarily focusing on calcite and dolomite, and their various forms and classifications. Key concepts include carbonate grain textures, the origin of carbonate rocks, and the effects of environmental conditions on calcium carbonate precipitation. The chapter also touches on carbonate diagenesis features such as stylolites and highlights the challenges of dolomite formation.