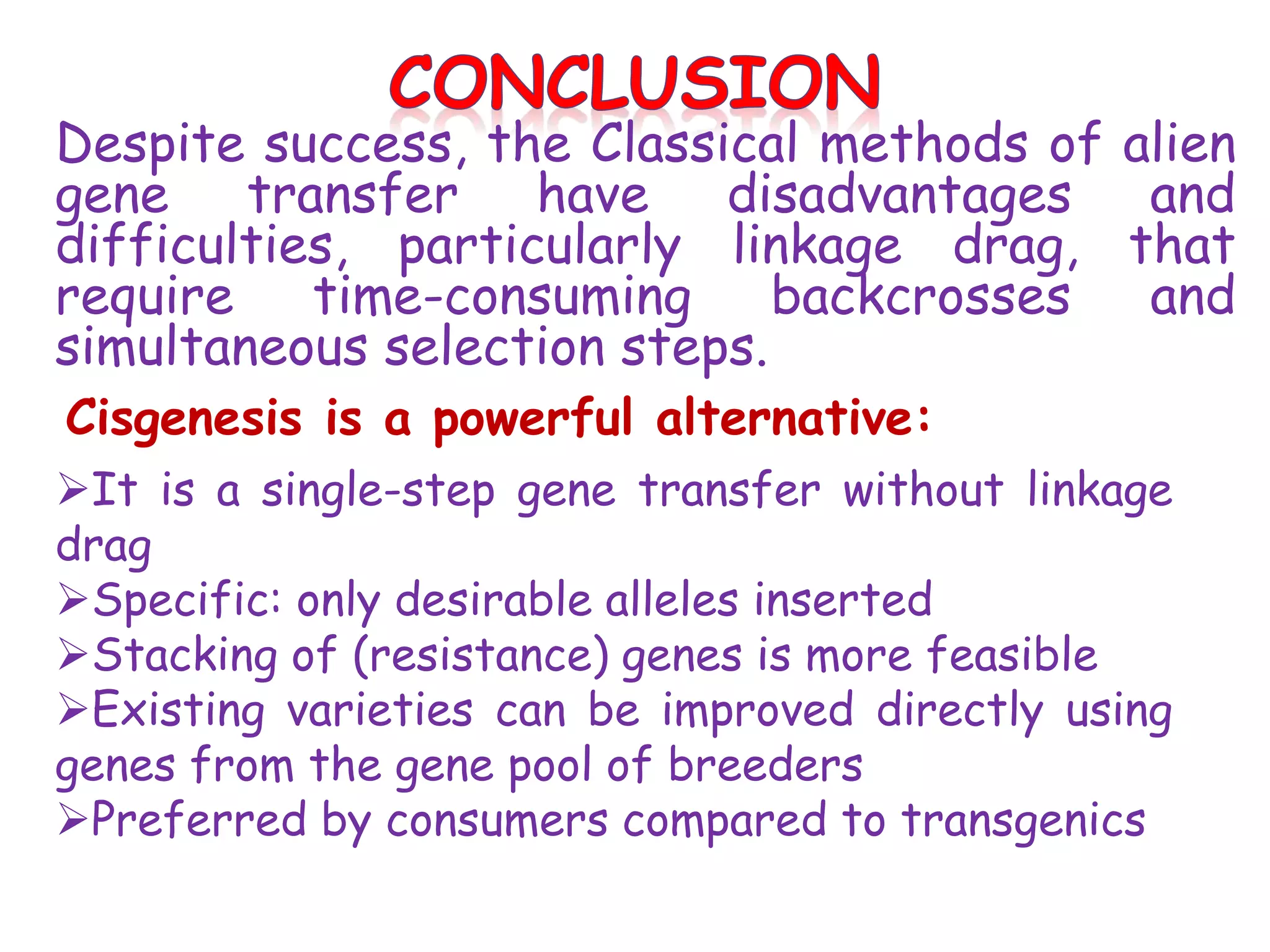
This document provides an overview of cisgenesis, which involves genetically modifying a plant with a natural gene from a sexually compatible plant species. It defines cisgenesis and provides examples of cisgenic crops. It discusses the limitations of traditional breeding and transgenesis, and how cisgenesis addresses some of these limitations. The document outlines the process for developing cisgenic plants, including gene isolation, vector construction, transformation and selection methods. It summarizes a case study on developing cisgenic apple with scab resistance. Finally, it discusses opportunities and challenges for the future of cisgenesis.