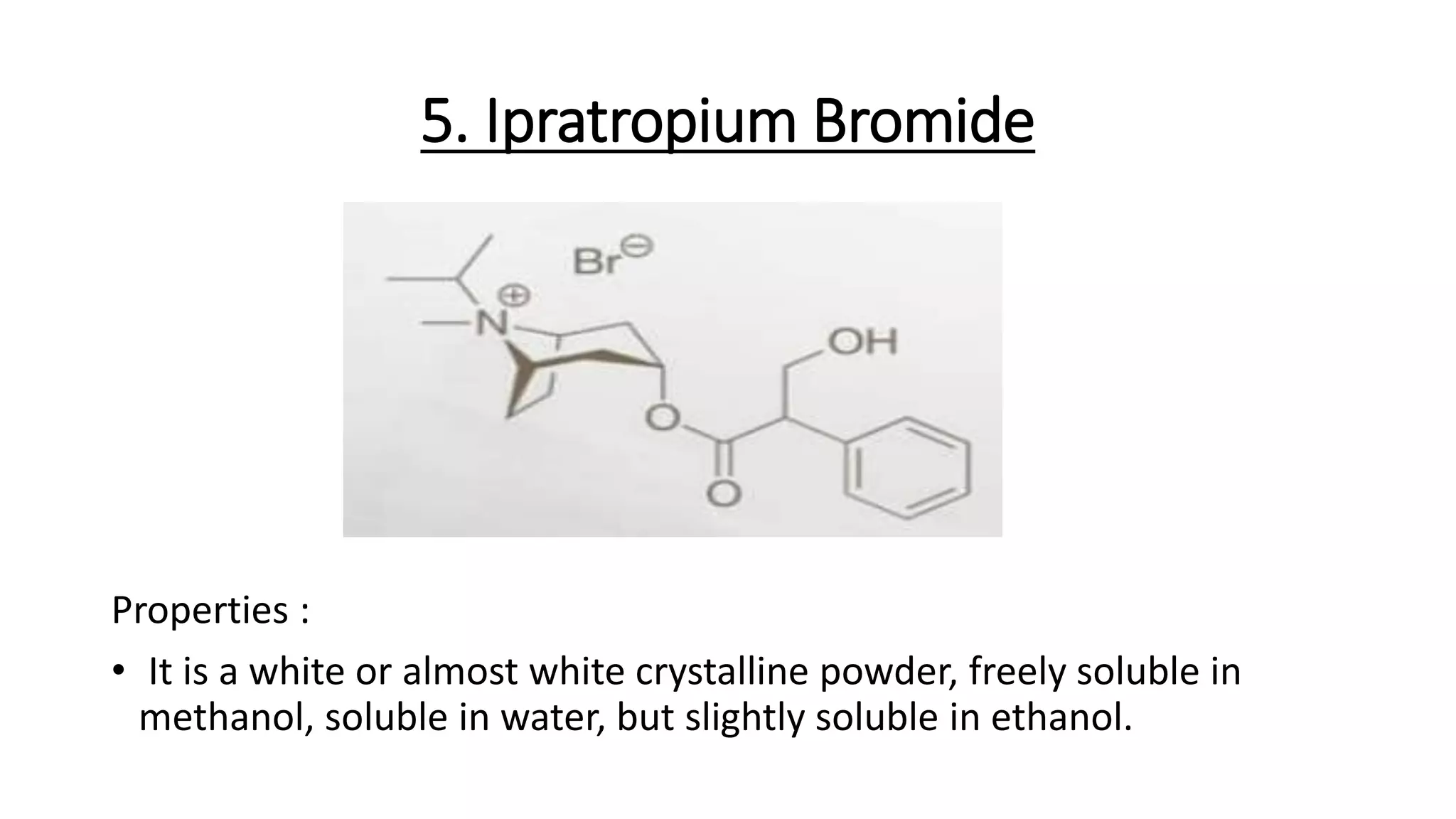
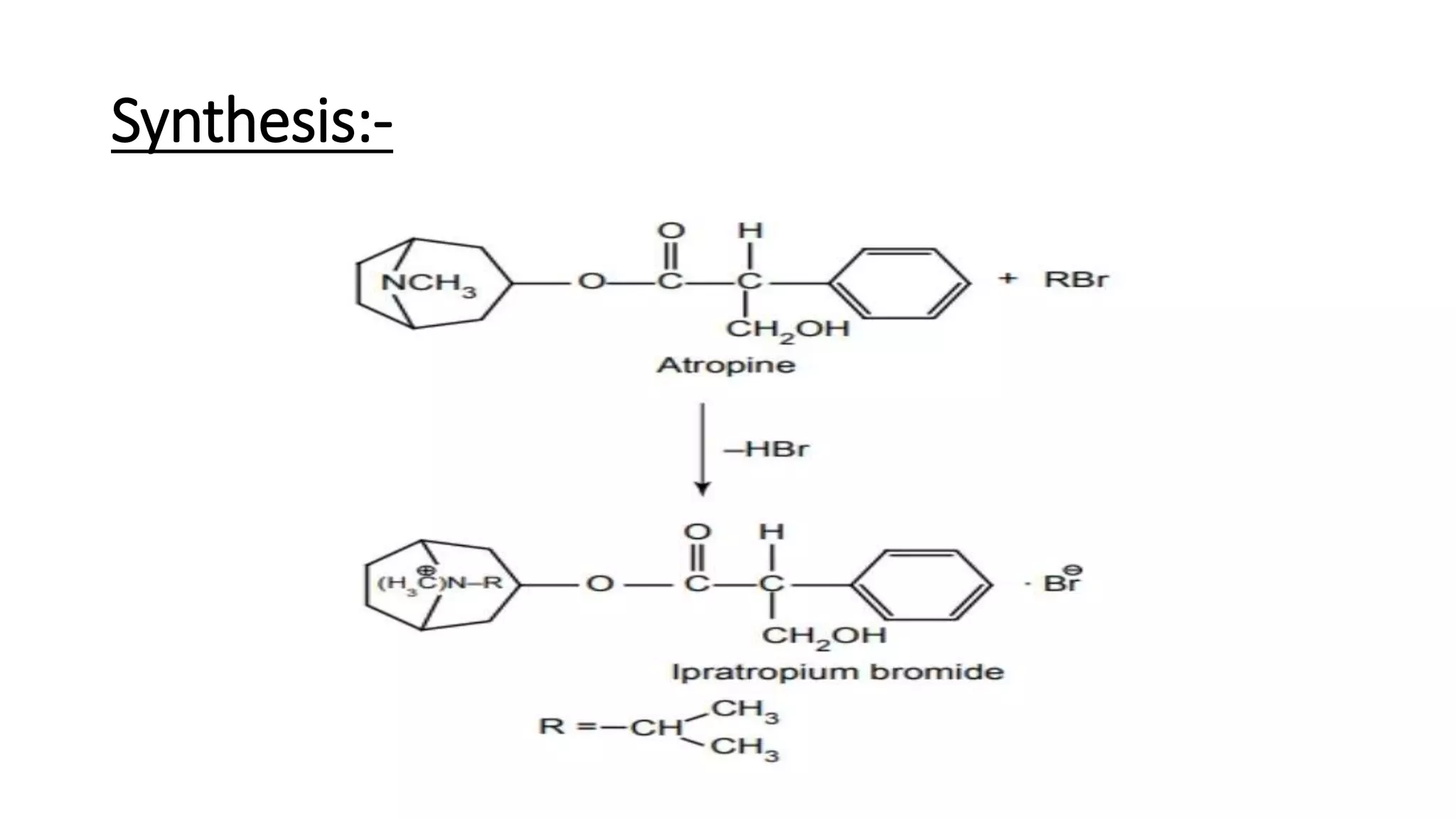
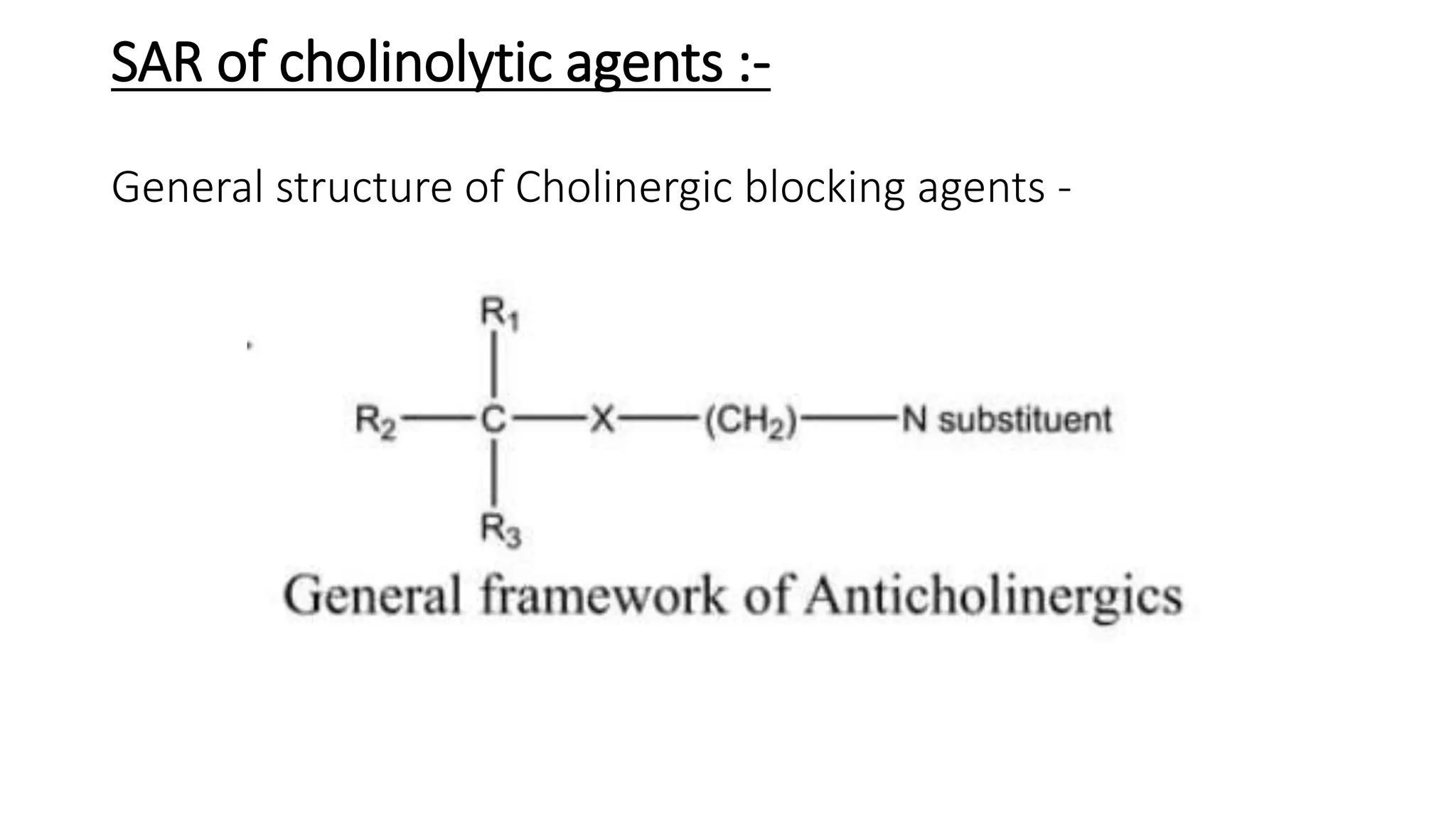
This document discusses cholinergic blocking agents, also known as anticholinergic or parasympatholytic drugs. It describes how these drugs block the actions of acetylcholine in the parasympathetic nervous system, reducing various effects mediated by muscarinic acetylcholine receptors like salivation, lacrimation, urinary incontinence, diarrhea, gastrointestinal cramps and emesis. Some examples of cholinergic blocking agents mentioned are atropine, propantheline, and biperiden. The document also discusses the structure-activity relationships and mechanisms of various anticholinergic drugs.




![Solanaceous Alkaloids and Analogues:-
The solanaceous alkaloids, represented by:
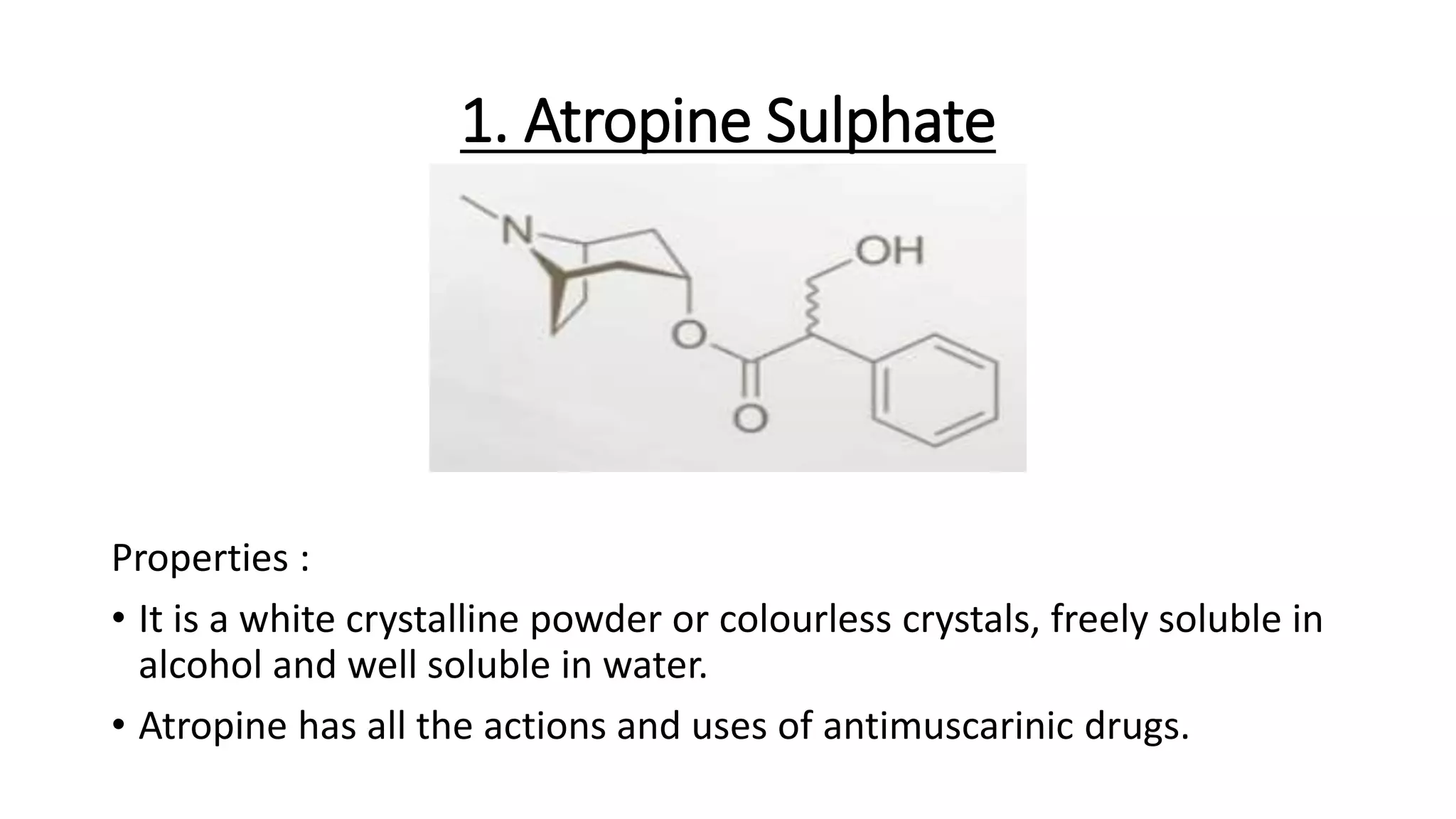
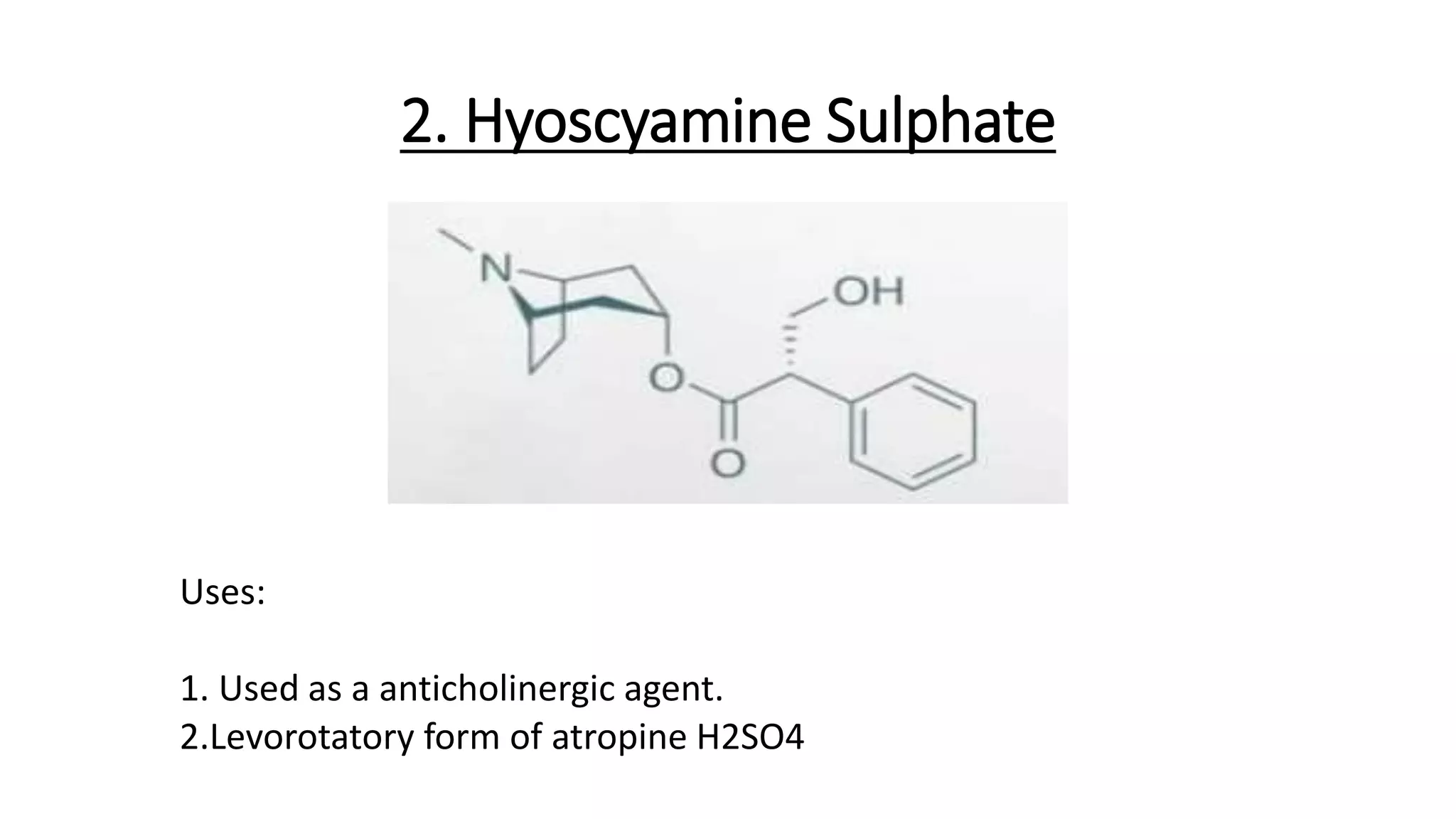
• (-)-hyoscyamine
• Atropine [(+)-hyoscyamine], and
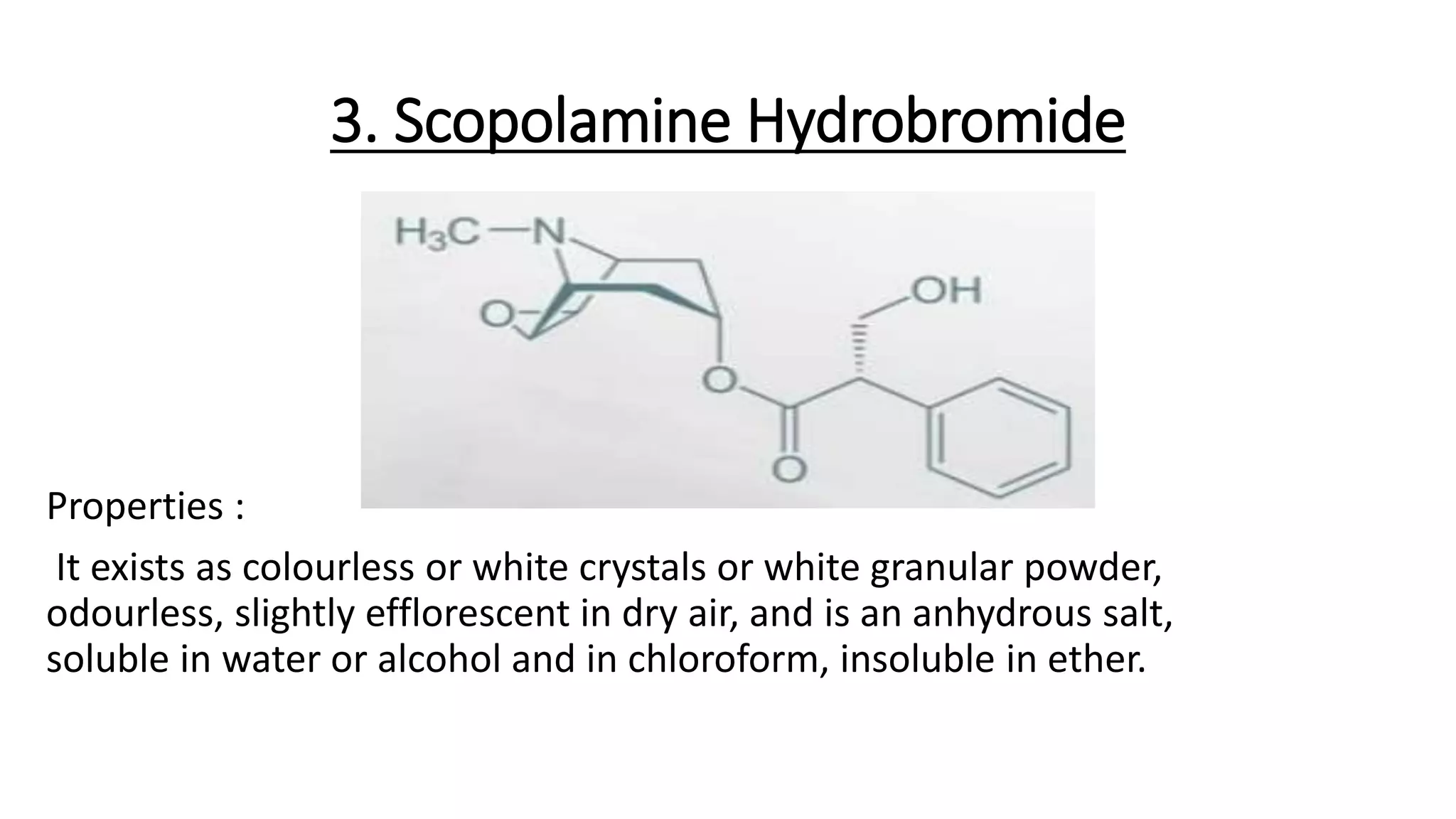
• Scopolamine (hyoscine).
• The forerunners of the class of antimuscarinic drugs.
• These alkaloids are found principally in henbane (Hyoscyomus niger). Deadly nightshade (Atropa
belladonna).
• All of the solanaceous alkaloids are esters of the bicyclic aminoalcohol 3-hydroxytropane or of related
aminoalcohols.
• Their chair conformation is accepted because this form has the lowest energy requirement.
• They were used in hemorrhoides because of their weak local anesthetic effects.
• They stimulate the respiratory center.
• They lead to mydriasis.
• They commonly use as antispasmodic.](https://image.slidesharecdn.com/cholinergicblockingagentsbyaryanpatel-230820082517-def1622c/75/Cholinergic-blocking-agents-by-Aryan-Patel-pptx-10-2048.jpg)