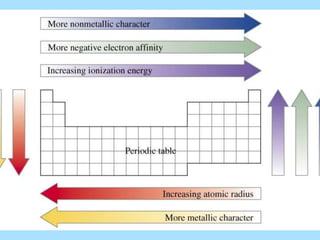
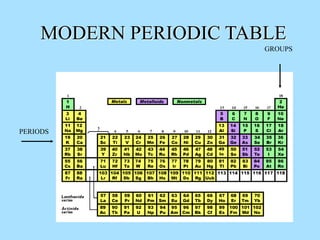
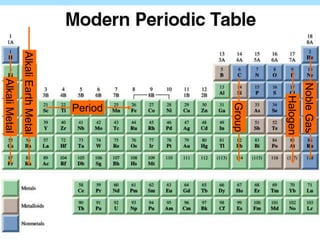
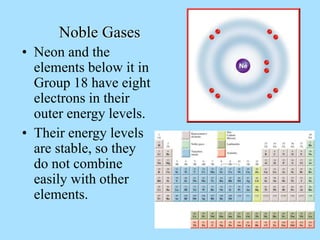
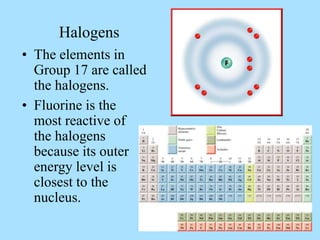
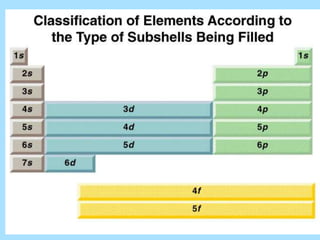
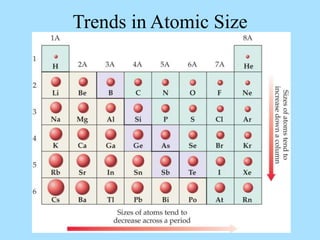
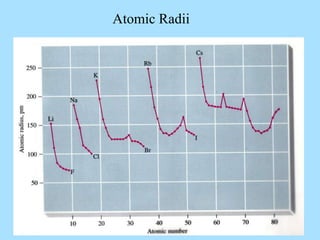
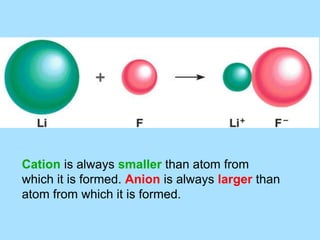
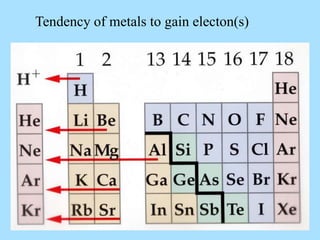
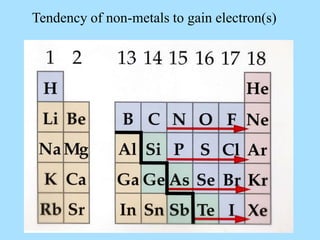
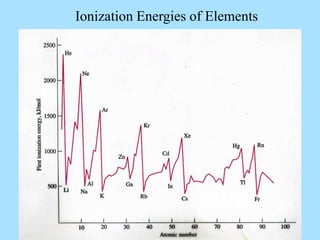
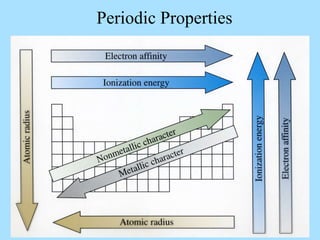
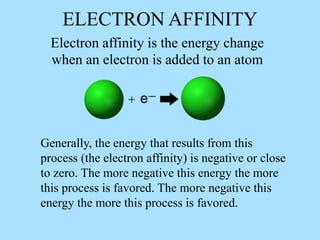
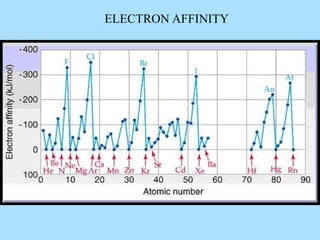
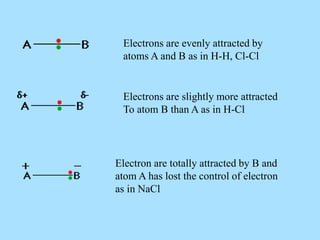
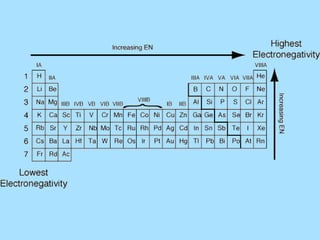
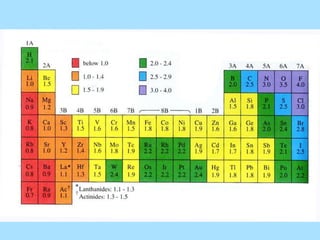
The document is a presentation on periodic properties of elements. It discusses how elements arranged in order of atomic number show recurring patterns of properties. These properties include metallic/nonmetallic character, atomic radius, ionization energy, electronegativity, and reactivity. The modern periodic table organizes elements into periods and groups, with elements in the same group having similar properties due to their outer electron configuration. Periodic trends are shown for atomic size, ionization energy, electron affinity, and electronegativity.