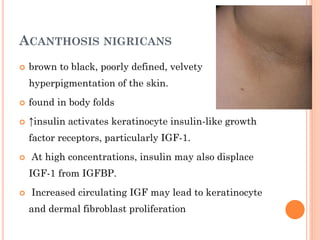
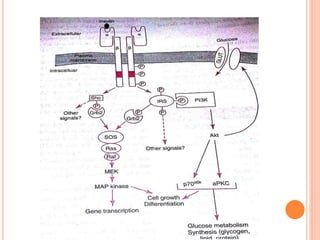
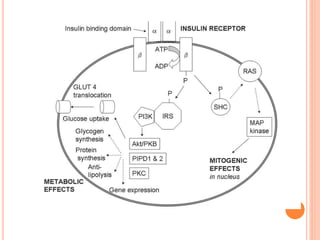
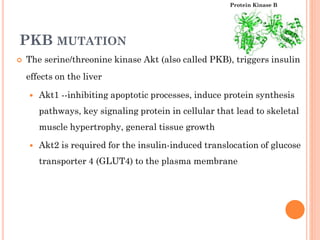
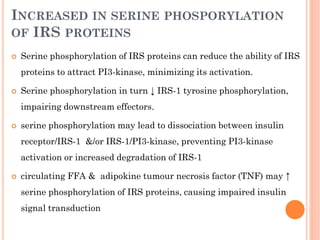
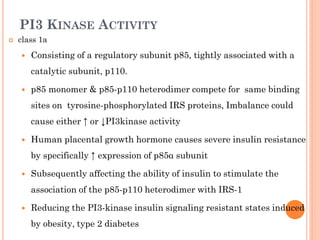
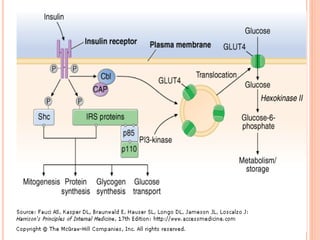
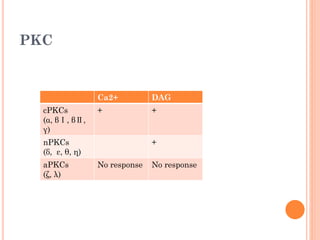
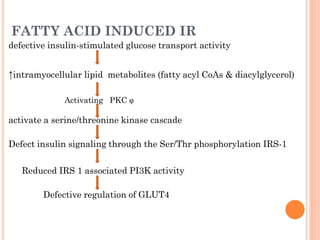
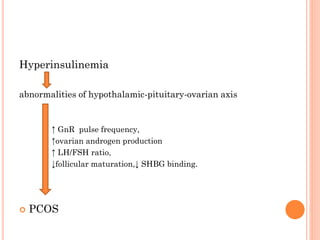
Insulin resistance is caused by decreased biological response to normal insulin levels and is often seen with conditions like diabetes, metabolic syndrome, obesity, and pregnancy. It can cause an inability to focus, increased hunger, weight gain, and high blood pressure. Insulin resistance is associated with increased insulin production by the pancreas and can lead to diabetes, cardiovascular disease, and other health issues. It involves defects in insulin signaling pathways involving proteins like PKB, IRS, and PI3 kinase. Treatment involves lifestyle changes and medications to lower blood sugar and insulin levels.















![MEASUREMENT OF INSULIN RESISTANCE
Research Methods
HOMA IR
= Fasting Glucose(mmol/L) x Fasting Insulin(mU/L)
22.5
Quantitative
Insulin Sensitivity
Check Index
(QUICKI )
= 1 / [log(fasting insulin µU/mL) + log(FBG mg/dL)]](https://image.slidesharecdn.com/insulinresistancecausesandconsequences15-131118084452-phpapp02/85/Insulin-resistance-causes-and-consequences-28-320.jpg)