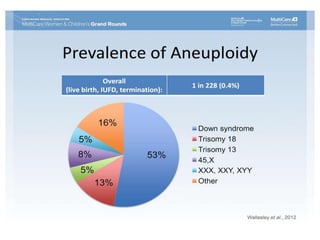
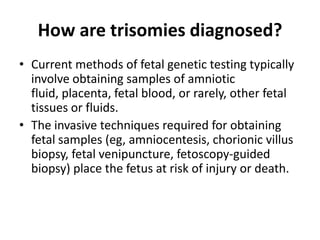
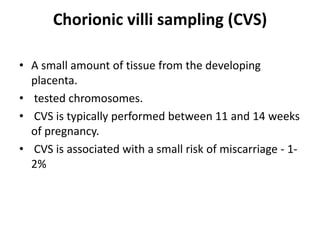
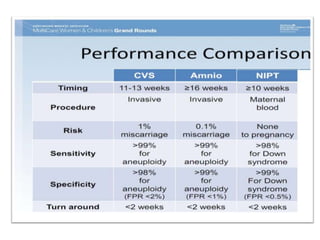
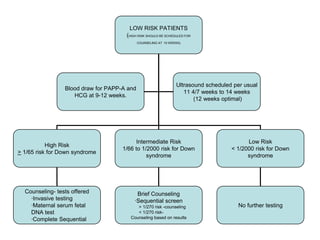
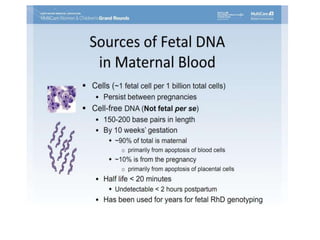
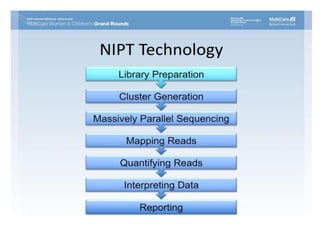
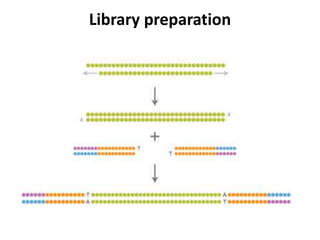
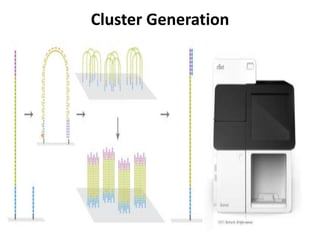
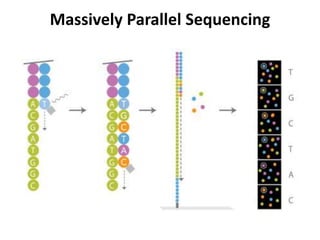
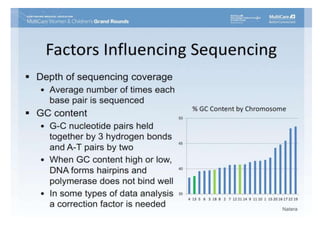
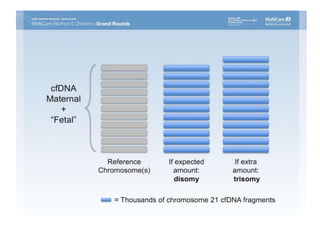
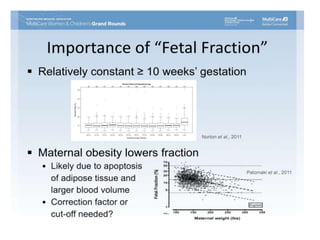
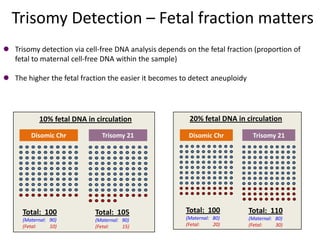
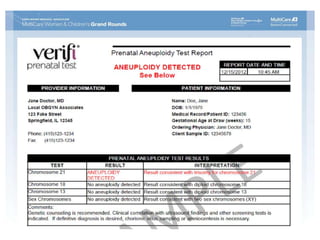
This document discusses using cell-free fetal DNA in maternal blood to non-invasively screen for fetal chromosomal abnormalities like trisomy 21, 18, and 13. Current methods require invasive tests on fetal tissue that carry risks of miscarriage. The document outlines how cell-free fetal DNA can be detected in maternal plasma using PCR and sequencing. It notes the percentage of fetal DNA needed for accurate detection and limitations like confirmation with diagnostic testing.