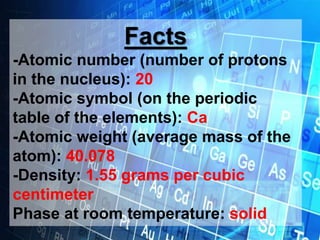
Calcium is a vital metal with an atomic number of 20, significant roles in biological systems, particularly in bone health. It is primarily found in nature as limestone and gypsum and has numerous industrial applications, including in construction and steelmaking. The metal is silvery-white and reacts with air and water, and its most stable isotope is calcium-40, which makes up 97% of natural abundance.