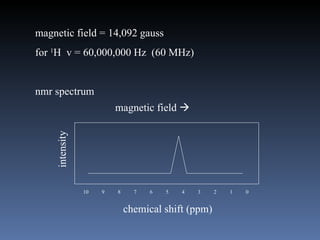
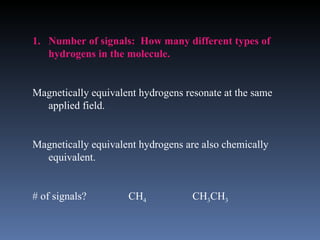
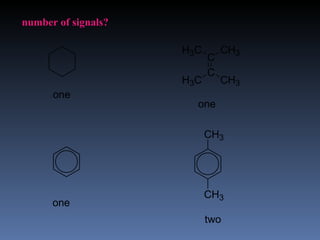
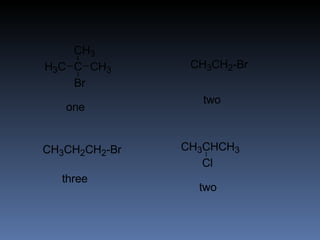
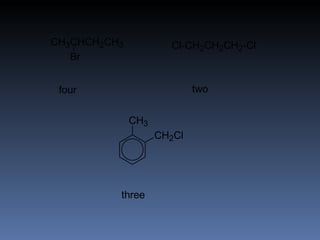
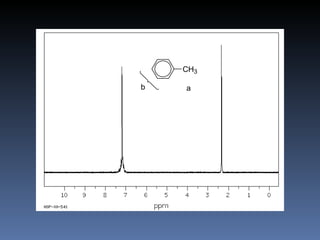
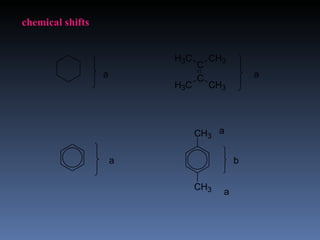
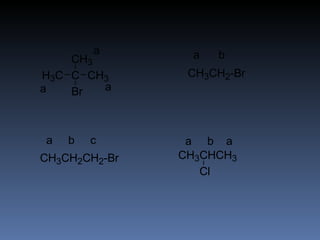
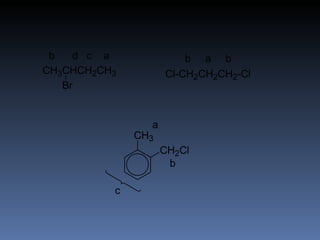
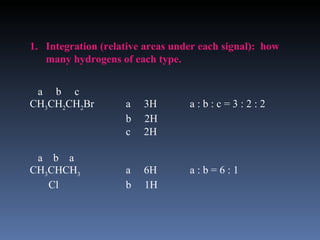
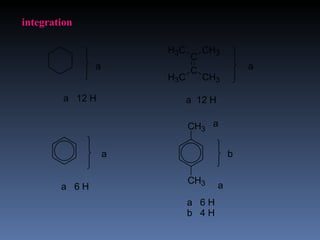
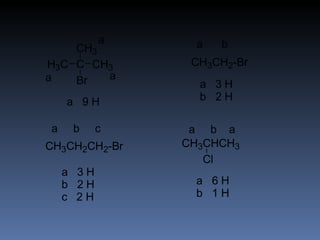
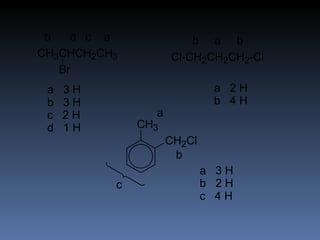
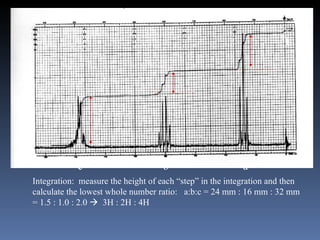
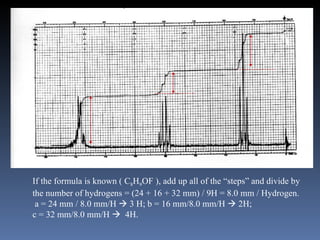
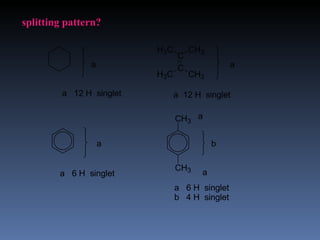
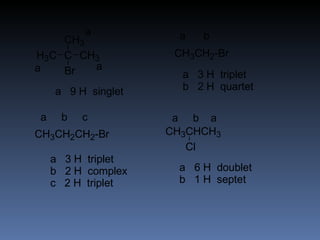
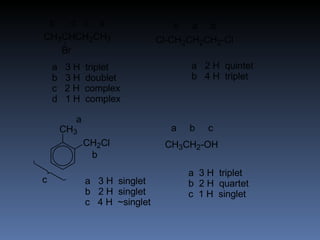
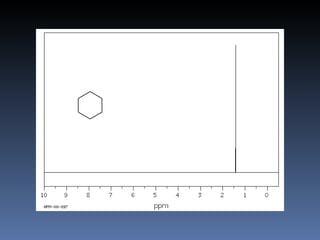
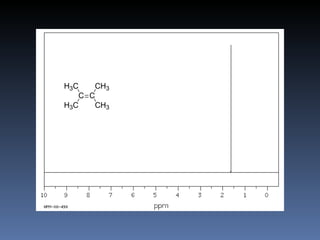
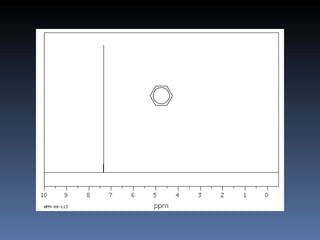
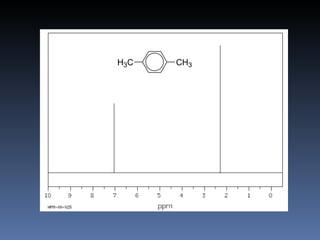
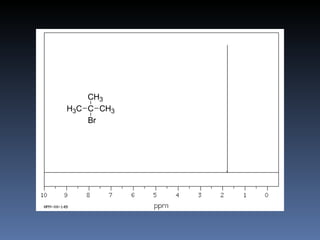
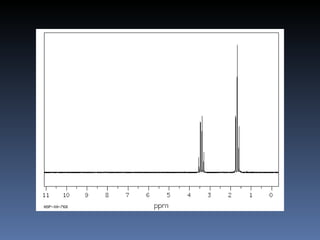
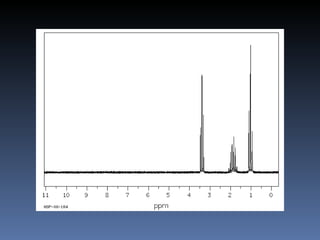
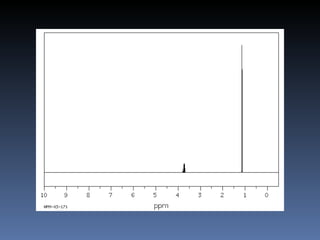
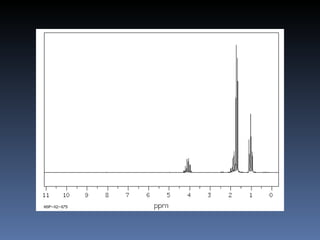
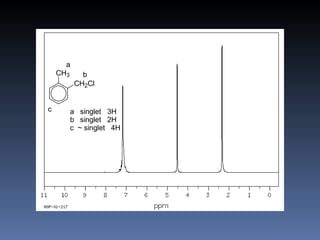
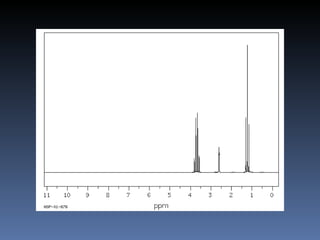
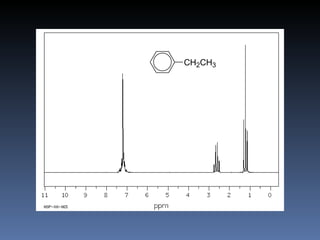
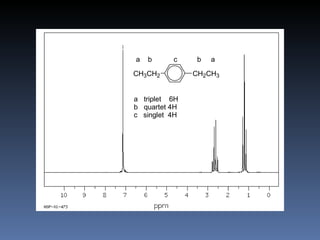
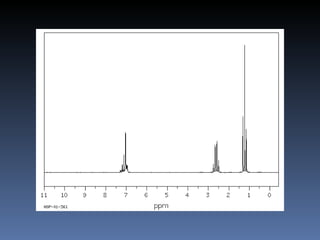
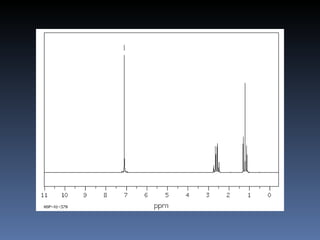
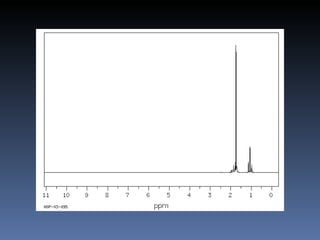
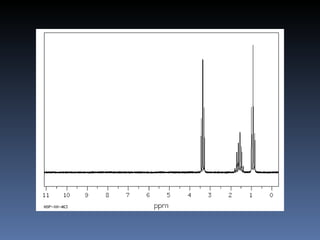
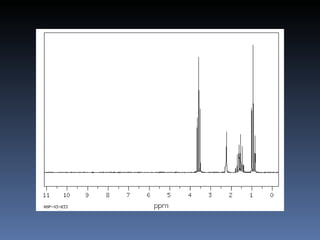
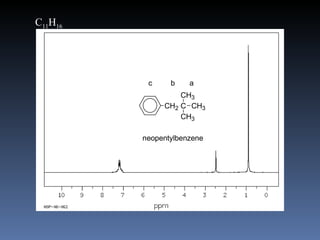
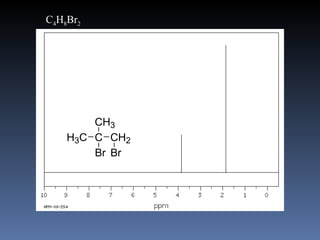
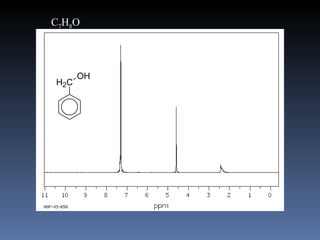
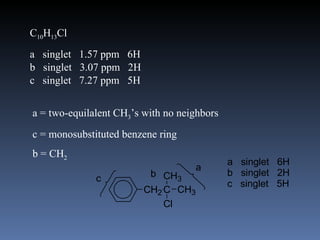
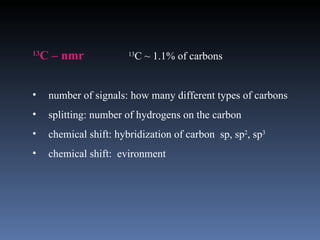
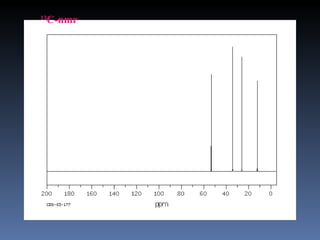
The document discusses nuclear magnetic resonance (NMR) spectroscopy and provides information about analyzing 1H NMR spectra. It explains that 1H NMR spectra can reveal the number of different types of hydrogens, their chemical environment, relative abundances, and neighboring hydrogen splitting patterns. Examples are given to show how these factors appear in 1H NMR spectra and how they can be used to determine structural information about organic molecules. The document contains over 20 examples of 1H NMR spectra and interpretations.