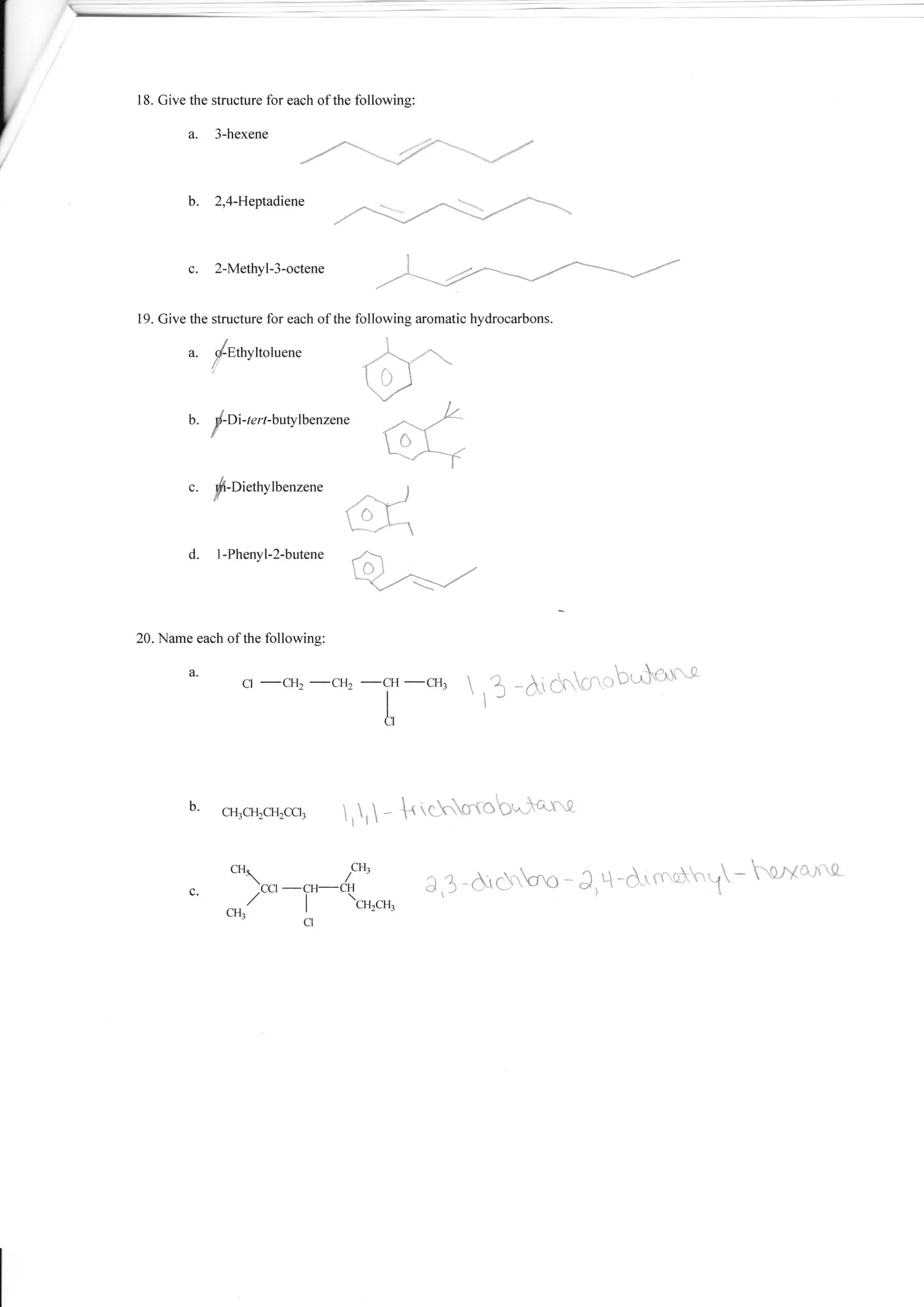
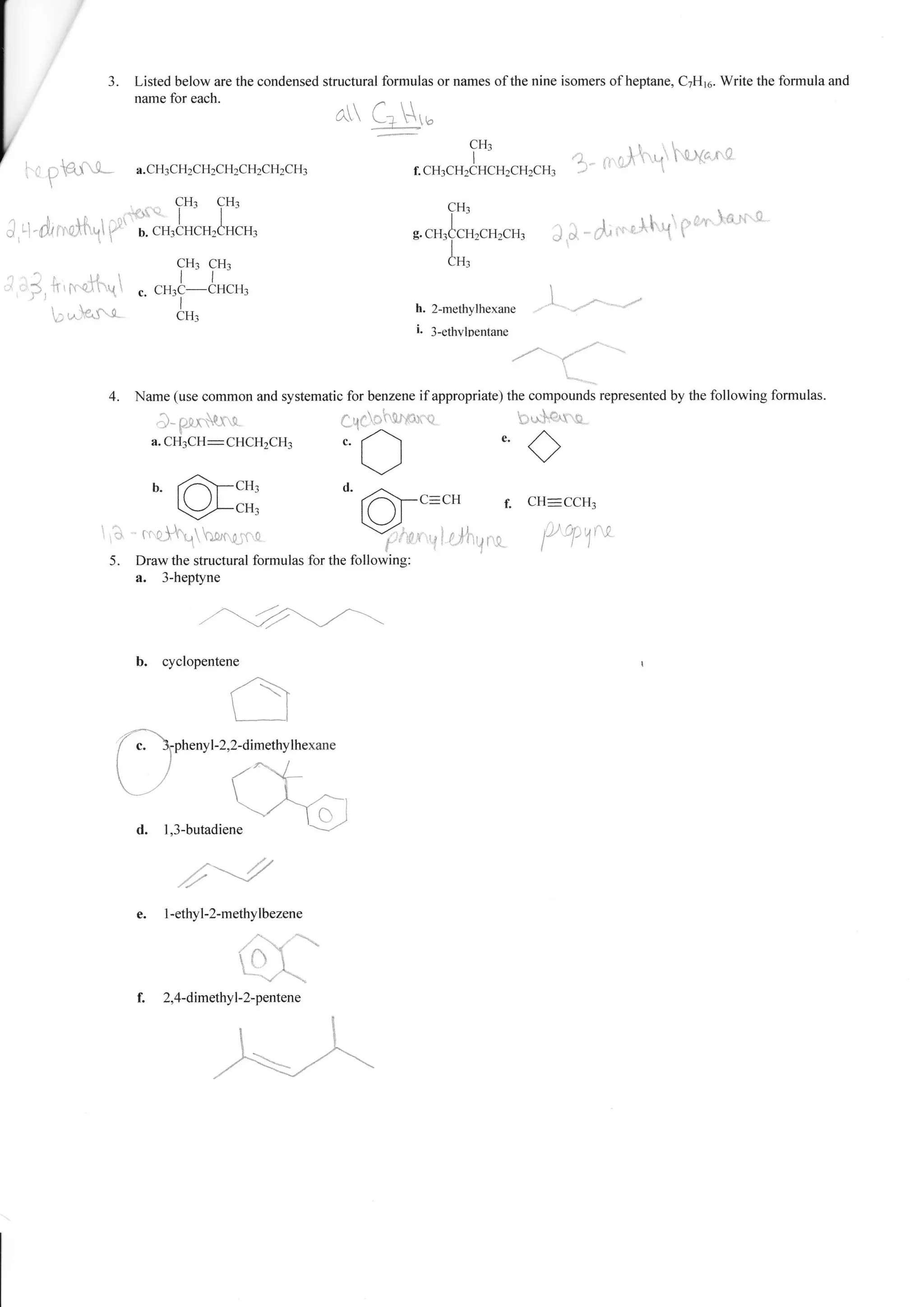
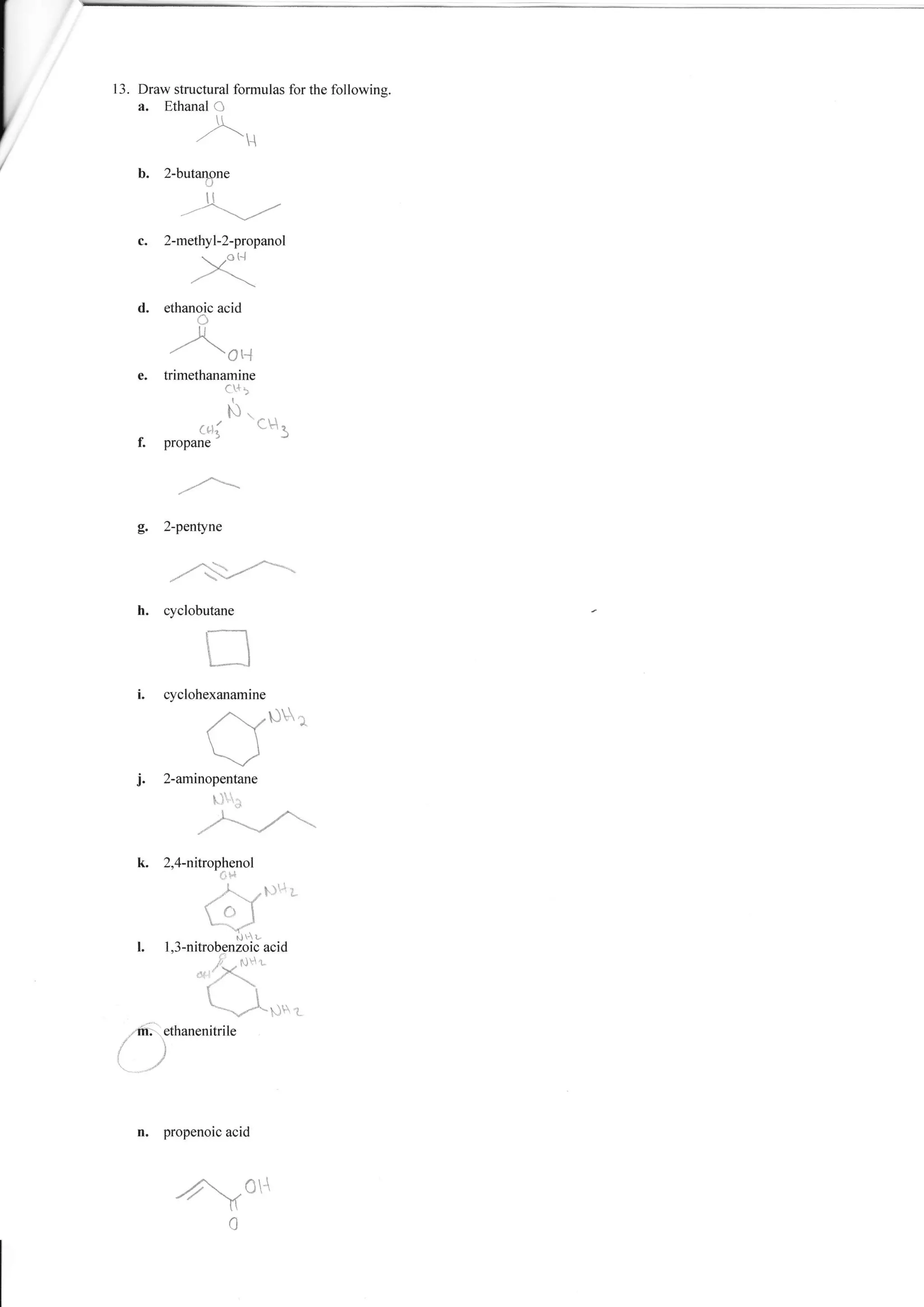
1. The document provides a worksheet with questions about naming organic compounds. It asks the student to name hydrocarbons, identify isomers, write formulas, and identify functional groups.
2. The questions cover topics such as naming alkanes, alkenes, alkynes, aromatics, and compounds containing functional groups such as halogens, alcohols, acids, and amines.
3. The student is asked to demonstrate their understanding of organic chemistry nomenclature through a variety of naming and structure drawing exercises.
![The Complete Organic Chemistry Worksheet
The Complete Organic Chemistry Worksheet.doc
Name
1. Name the following hydrocarbons.
3 j'- d m.u q'Lxc.$'s
i"'a.CH3-CH2-CH-CH-CH.
I
CHz
I
CHr
CHr
A A -k ,e*qt^'-tt i"' f"l-'"'-lu*",Uo..q b cur-f-is-a",
ll
"'
cH:
?"
3 a-O,trtrc$nt1t
c' cHl-C-CH3
b*fa^q- lt'CH:
. A CH7-CH2-CHJ
4 ,3
- /.eltrt1Ii'*-lt'asq,n,-"r',-J
'cH2-cH3
cH2-cH3
2. Name the following hydrocarbons.
J ^thl[r,k*
CHr
t"a.CH3CH2CH2CHCH3
9H,
I
b.CH3CHCH2CH3
CHI
I
c.CH3CHCHCH3
I
CH2CH3
d'n*{l'1lltnk, t
CHr
t-
CH: 9n'
tte. CHr-CH-CH-CH-CHl
I
CH:
CHr
I
?"' !t'f. CH3-CH2-CH-CH-CH
CHr
CH:
I
CH2
I
g. CH3-CH-CH-CHr
is-cHr-cH,
I
CH:
CHz-CHr
I
h. cH3-cH-cH-cH3
I
c+r3-cH-cH3
d.CHICHzCHCHzCH:
I
CHz
I
CH:
g'ulktlfe J-o'"L
'l.3')'ont'lr,,lyW
3'ml{f ynh'^t
f"' fn' ) u (A nolfu''{ ttu'*u*'Qe.CH3CHCH2CHCH3 cJ 1{
,. .,.[X1".","", a,, - &*]f'f gw-fu{"{
cI{3
A,r'd.'*uf $'
tf W^u*g.CH3CHCH2CH3
I
cH,
l'CH:
?',CH"
l'
h. cH3cHcHCH3
CH;
f, ,3,
q - c' *"jtr1-s.x^t'"*-
j - A.^ r.alh-{hq"{n-"*
3 ;1,
Ll
-.lr iu .t"1ihqaru-
Z, 1, q * ,,0'ah.1F *+ra*r's](https://image.slidesharecdn.com/completeorganicchemistryworksheetanswers-150325231347-conversion-gate01/75/Complete-organic-chemistry-worksheet-answers-1-2048.jpg)
![6. Listed below are the condensed structural formulas or the names for the eight isomers of C5H11Cl.Write either formula and the
name for each.
,/
'1'l "*-'f:1:P''
:'1e'5 '';
a. CH3CH2CH2CH2CH2CI
CHrifil
. ttr fli,- . !:r'"V'1
b. CH3CHCH2CH2CI ';_;1a$;q.g
Fr
' c.2-chloropentane
io;;f
d. 2-chloro-2-methylbutane
7. Name the following compounds.
CI
I
e. CH3CH2CHCH2CH3
?", :- 1 i, ,r , ij "r - 1 , c.,1 -
I
a. CHTCHTCCHTCHTBT o i. .., f . lx !*- -l f. i
CHr
t-CHr
j -crt'c,-'b [.,];
si ";
CHI CI
i--' i--^.- 6 -e ''c"n '3-rru'*L?h '''is^rp
CH3CH-CHCH3
l-chloro-2-methylbutane --.-*){
l'---i-
* -I -chloro-2, 2-dimethylpr"?ri_,-X:
CHr
i--1 r - rro*h'{ * }' -
P'tx'':k*eh. CHTCHCH:CHCH:
CH. CH"
' ^'' I
-
l^:' ^" ! 't' -d t {-' .'}ri
"*" Pa^nffrti.CH3C:CCH2CH3
t_.-t,. .-" ".
r
j
'{ .nn/
{t
i
f.
g.
h.
. ^rr ^,,i1,,
+r-qN)n1- 1,{-par{adt**'0,.^..
il, i" ^-- ) , A...ah.i - ,'4 - prr*&re:e'*
5. CHr:CHCHCH-CH, t'CHr-QQgzC-gy1rc',-' ur'
CHr
I
c' CHI-CHCCH3
I
CH:
I O..l 2-methylnaptJralene
!; ir'd,i,*s''ia - 1-bu'knc
!', f",k.cHr-c-c-cH-CHCH3
cHr
rBr
j,3 ,'i-lr'n'r),hql - 1, t -
h''wa.15 l{,u
d.
e.
c6H5cl akot dCli', }l'r)
CH:CH:CHCH2CH3
CHr
I
f. CH3C:CHCH3
g. CH3CH2CH=CH2
Draw structural formulas for the following.
a. 3-heptene
8.
trichloromethane n Ftf
.1't li
d. 2-chloro-3-phenylhexane
^
,^_,_. .,..
-" -Y
e. 1,3-cyclopentadiene
{i1
_-
L)toluene (methy lbenzene)
g. 1,4-dibromobenzene
tlt :...tls,
h. 2-bromo-3-methyl-2-butene
tJ, i
.'.rt](https://image.slidesharecdn.com/completeorganicchemistryworksheetanswers-150325231347-conversion-gate01/75/Complete-organic-chemistry-worksheet-answers-3-2048.jpg)

![10. Name the following organic compounds.
i, , I
,3'r. li-1 l- a.cHrcH-cHCH,cHl
,;p,"'^Janol
-J",
J"to
b. cH3cH28cHcH3
d. CHTCHTCHCHTCHT-l
CI
d. cH,cH.cH"c/ :l-^Inr n!*.
H
?n,
e. cH3cCH2cH2cH2oH d,J - Ah;lvt- 1 y^,hrt'"?.
CHr
rrt ru ,#'{iyi- V-y:e4 $e.rur,q J", ?''
r. cH2:6slg11-cHCH3
5-ftU.jf,,rf * ,q - tW*$,Un* -'1-
""q-OH
c. CH3CHCH-Q!{2
3 n'ofYr* -:L - b"j"r*
11. Name the following organic compounds.
a. CH.CH"CHCH"CI
-Ch{oo -X -nutt- q I
-1",
L,^)+rl.Q i"tb. CHTC-CHCH,BT
J-$q,rr'r-d 4,j-.krrnxlhq i 1", J",
bula$L
CHr
.. cH,EcLcr, 2.- rnolhq*a *b{r-qrrs'
"l
OH
r. cH3cH2!:cHCH3 !-h *,t**0 - qurnefil-
Br
". A s. ,rmnO*tp':?roflr*.
g. CH3(CH2)2CH2NH2 buJqAfr",rfS.
h.H,N-[-cH2cH3
p/Wcr((^&-[-
c. C5H16
Cl, - Cu. -K -- Ctr - c L{
35d
pj{.,.k {.,0*
t
*!j
f g!.ogl"'r"le*'u
rr1pflt6r,ywill*xr d-pt',pr','*r'a
3- ci".*,crPn'e"na
12.Each ofthe following formulas can be written as two compounds with dif[erent functional groups. Write the structural
formulas, name the compounds, and identify the functional groups.
c]r'C,ilr - c,f+
MaryTL P^cff
rbl
a. CzHoO
eu"-al^-0F- 5 *"d
b. C:HoO
0
ll
a
[
nu^- t-LH.v"5 -)cHr- o -cll3](https://image.slidesharecdn.com/completeorganicchemistryworksheetanswers-150325231347-conversion-gate01/75/Complete-organic-chemistry-worksheet-answers-5-2048.jpg)
![14. Draw and name the five structural isomers of hexane (CoFir+)
Q nilhllyntiarn
'/o'
cH2cH2cll
J,b)5--|r,ur ddrl -
8-dt1dscq,.rI
cx{3
-c -cHI
cH3
o,
2cH2CHCH2CH
I
cH2cH3
CH;
b.
3, 5 - A^d.aj - 3 - 'aqRl'*
3- r'nqrh1 h-ue'"o
17. Name each of the following alkenes.
a.
CH, :611
-(]12 -CH3-4.+-
L- buJ'[fa
cll3
c. I
I
cH3cH2cH-cFI-CH-Cr{
/"r
o,
'3l'nCf'qlk $qj.tq
./ -J,Q - d''
^.tJ
[t I
)<----/ D',ier
i. orlanq
15. Draw the structural formula for each of the following.
a. 2-Methylpentane
,
-/A------//^-
16. Name each of the following:
a.
CHT CH:
llcH
-c -cH2cH2cH3
ttbH, cH:
),i,3- rr m'dh'1 htxaru
C H3---{H2--{H2-C H
-C Hj
I
clI2_cHj
b. 2,2,4-Tnmethylpentane, also called isooctane. This compound is the reference for octane ratings for gasoline.
,/
X- -/---
-/--
c. 2-tert-Butylpentane
d. The name given in part c is incorrect. Give the correct name for this hydrocarbon.
0 ,
!, i - -h taru"I'h'1 ha"v.c$-tl-](https://image.slidesharecdn.com/completeorganicchemistryworksheetanswers-150325231347-conversion-gate01/75/Complete-organic-chemistry-worksheet-answers-7-2048.jpg)