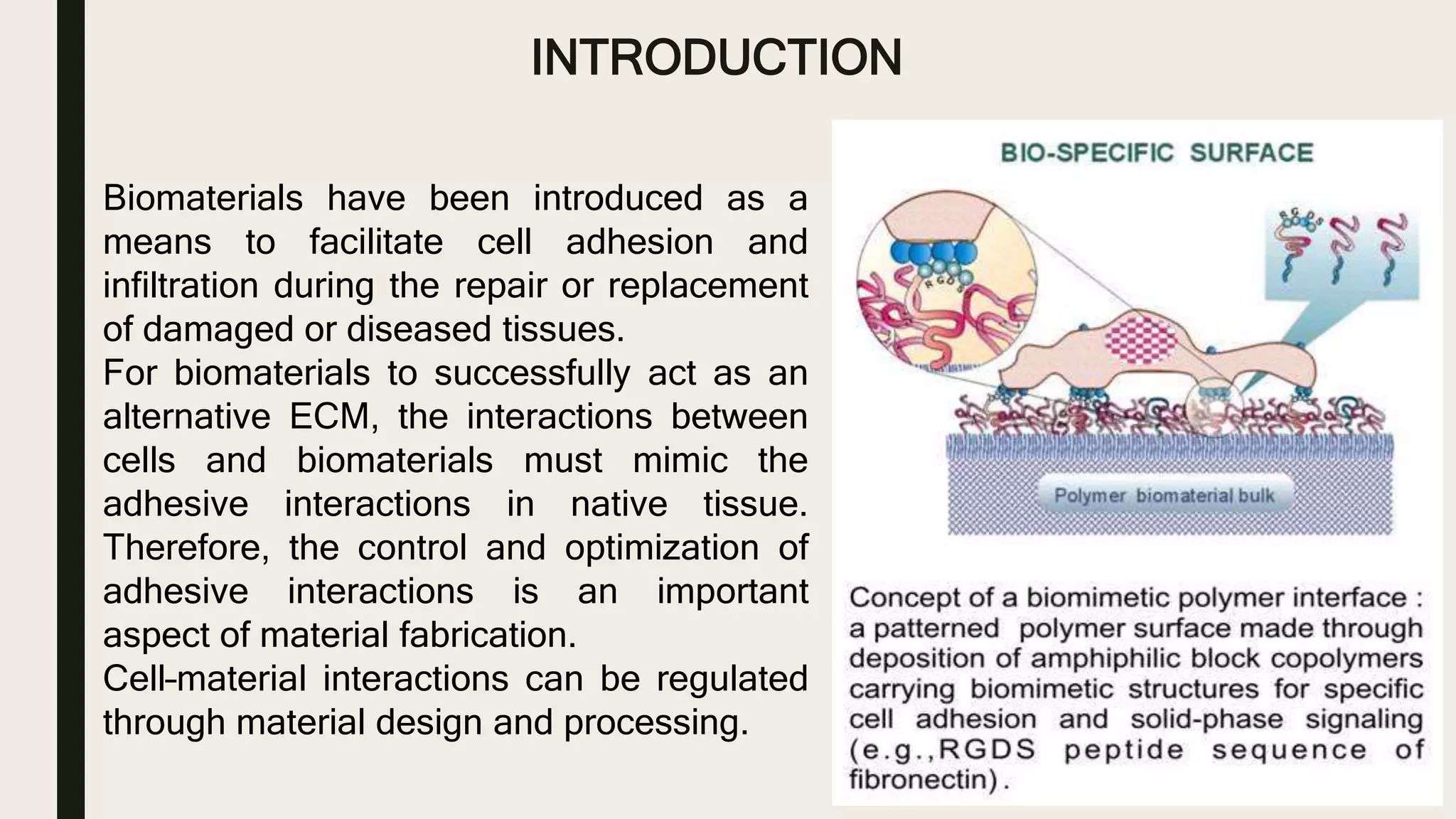
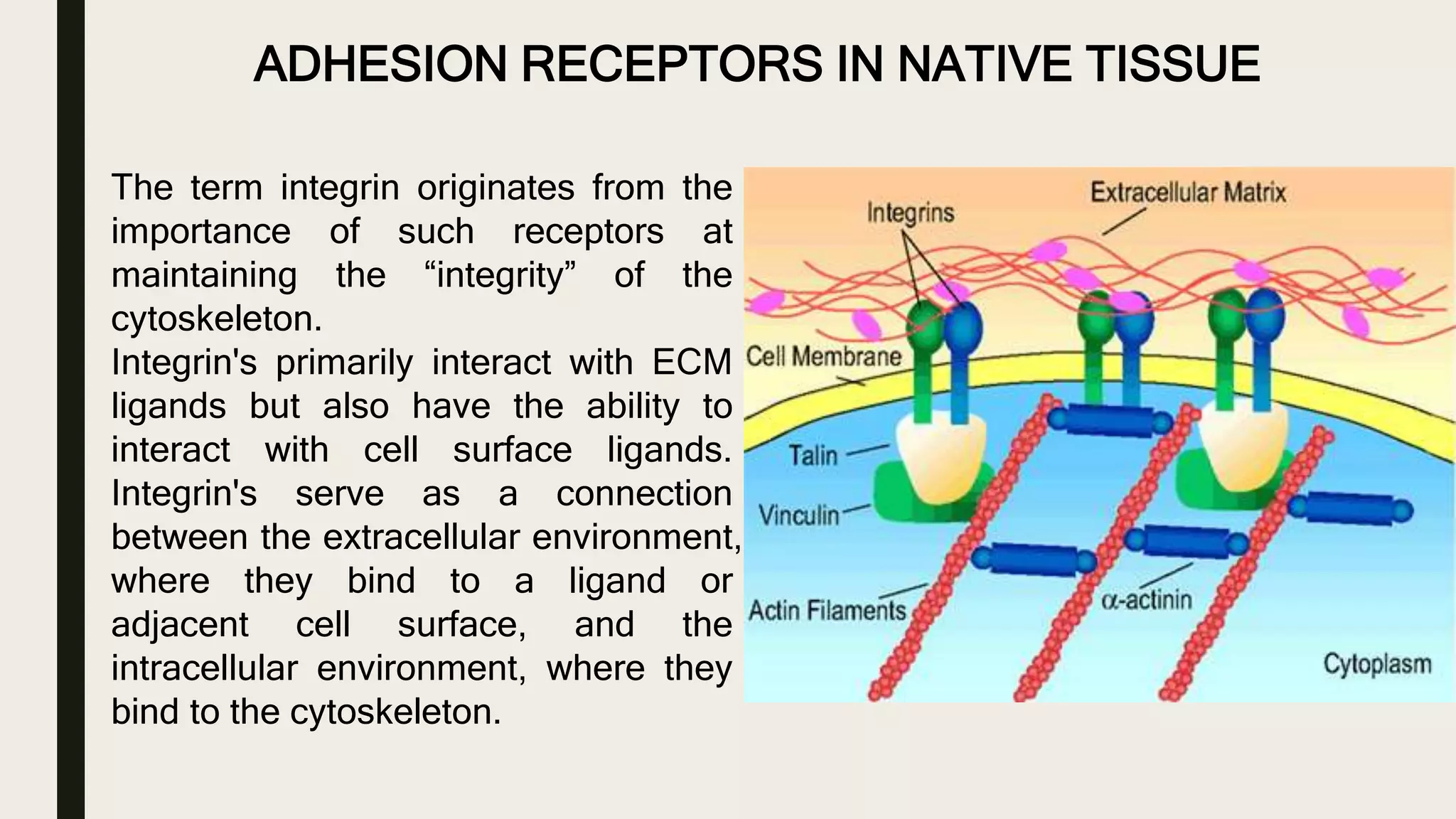
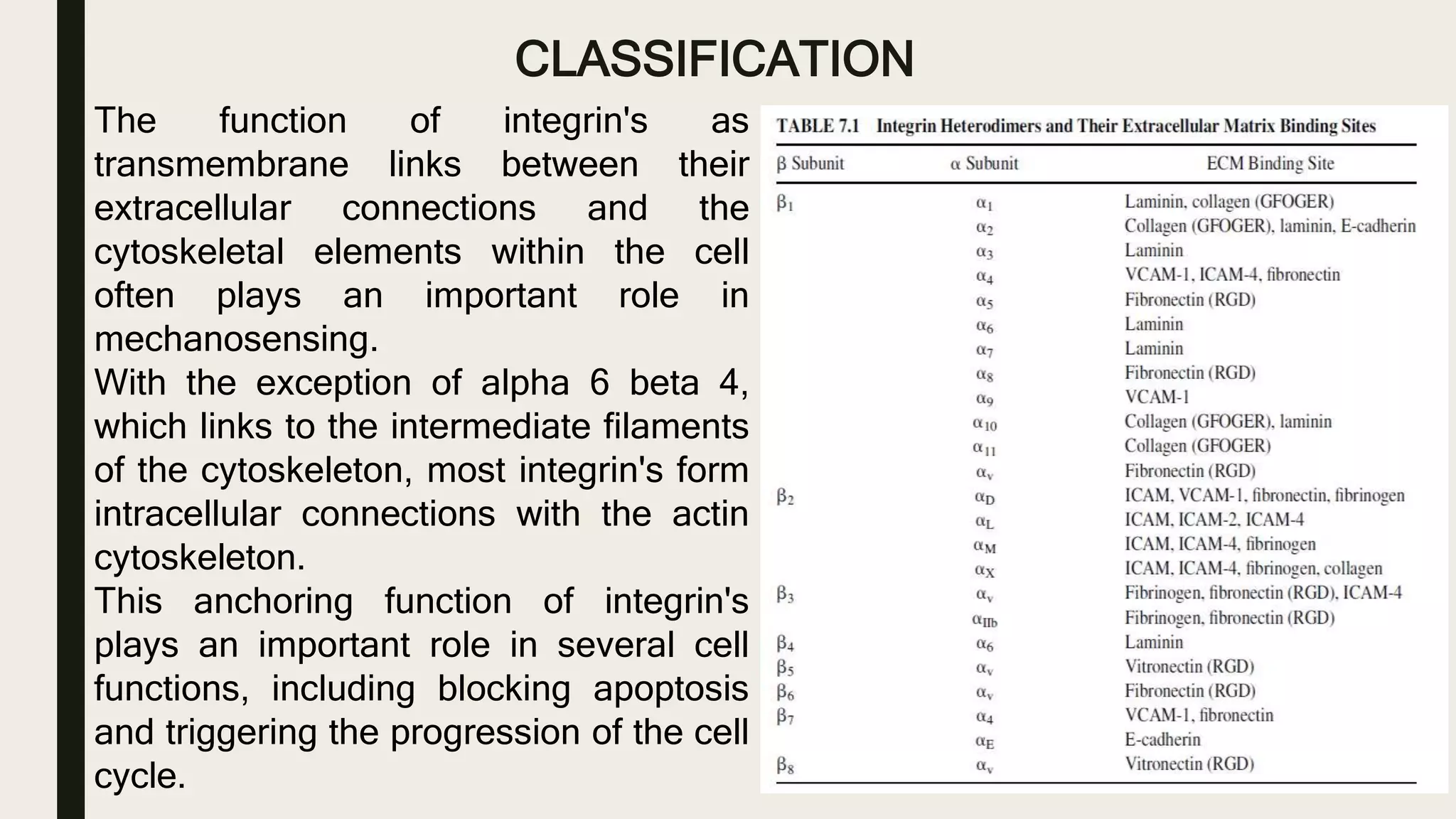
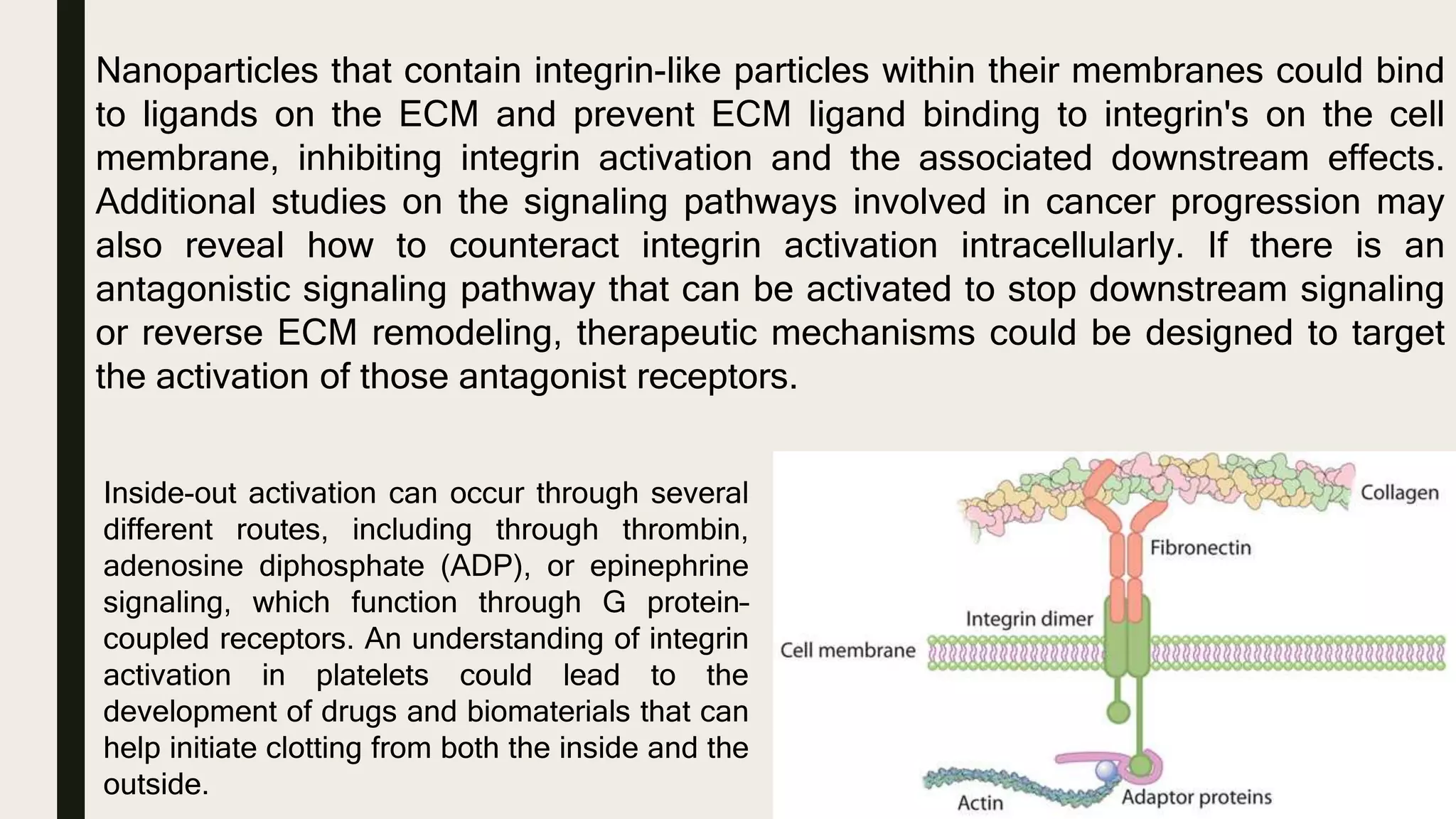
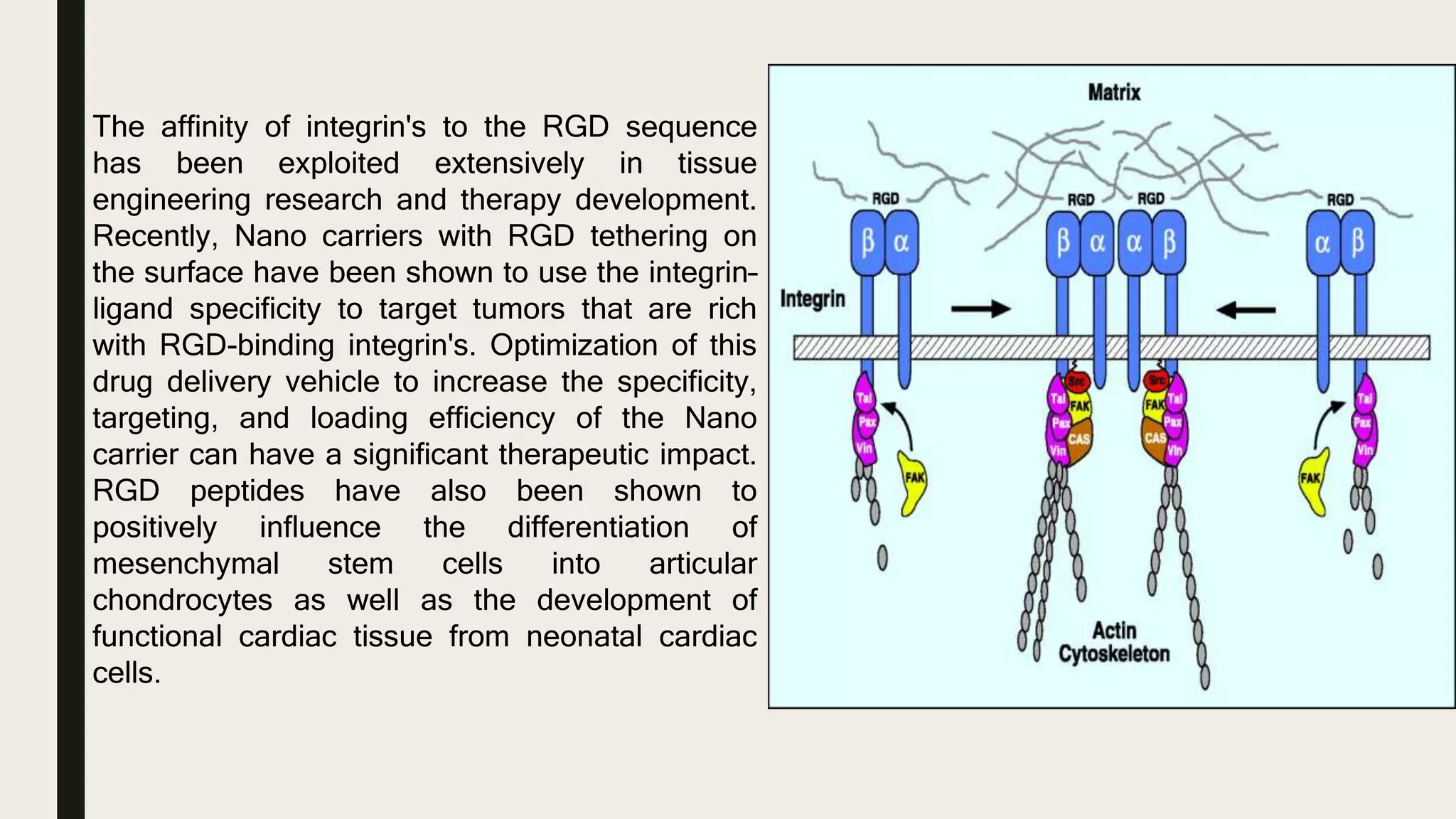
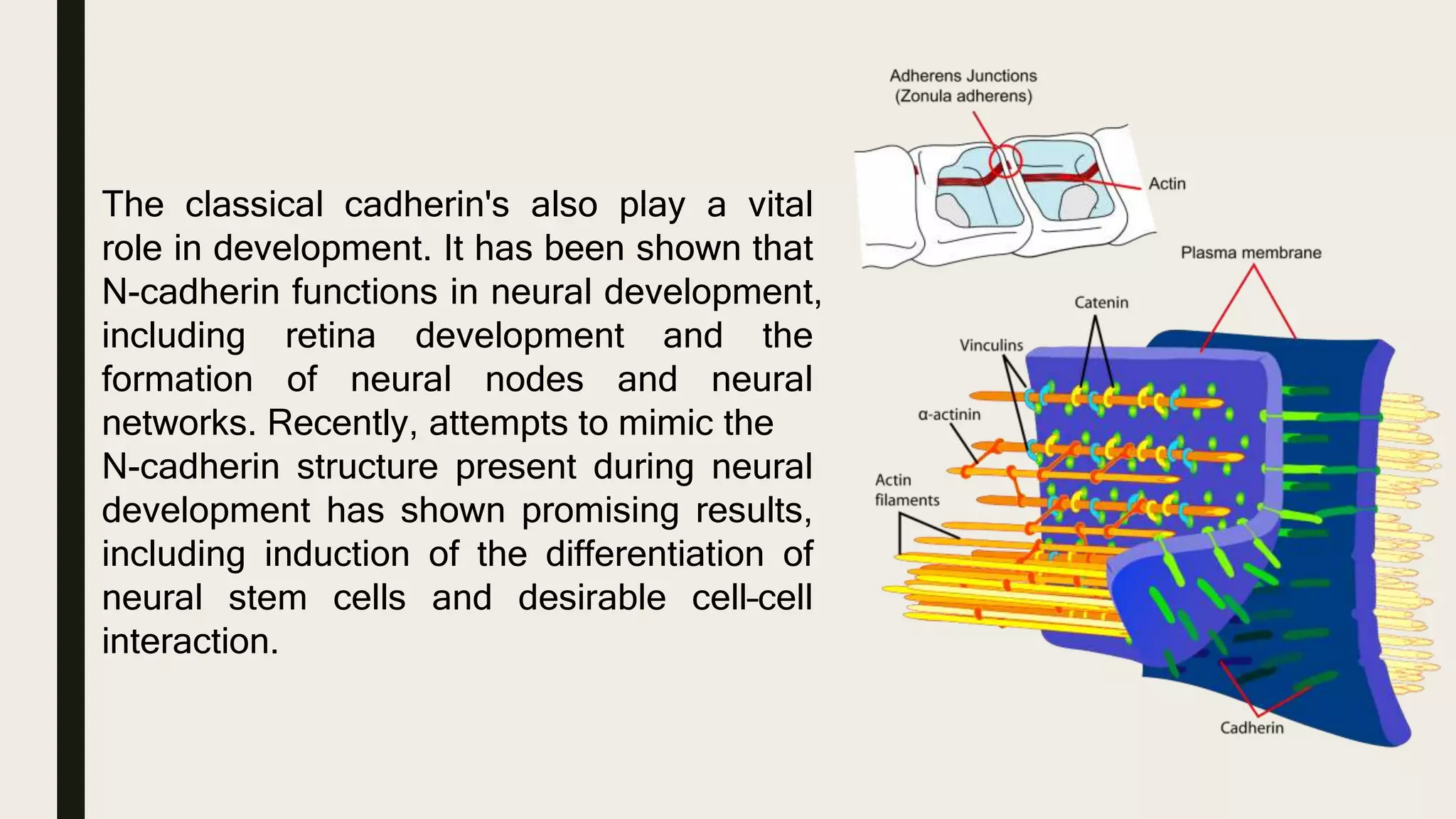
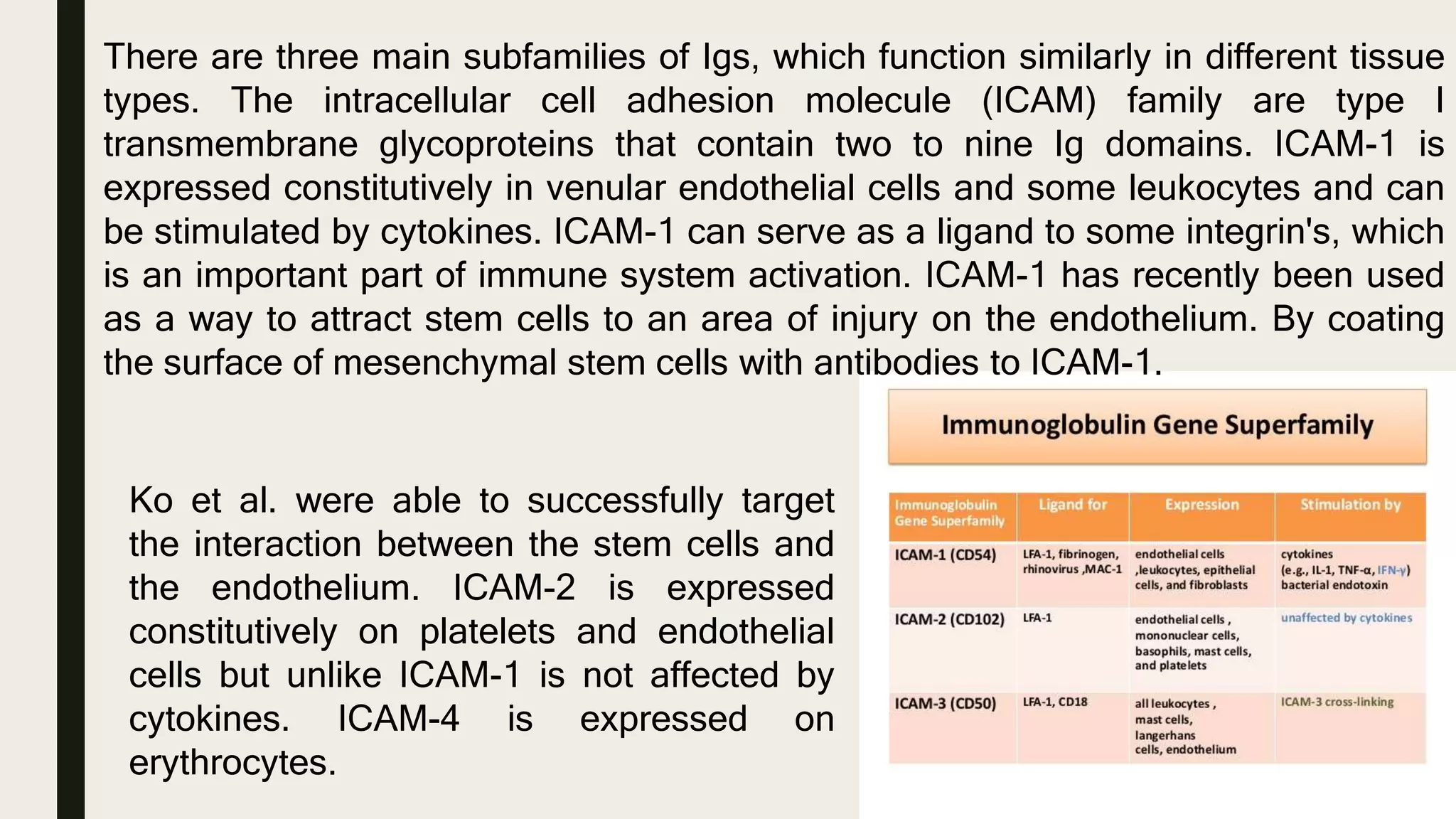
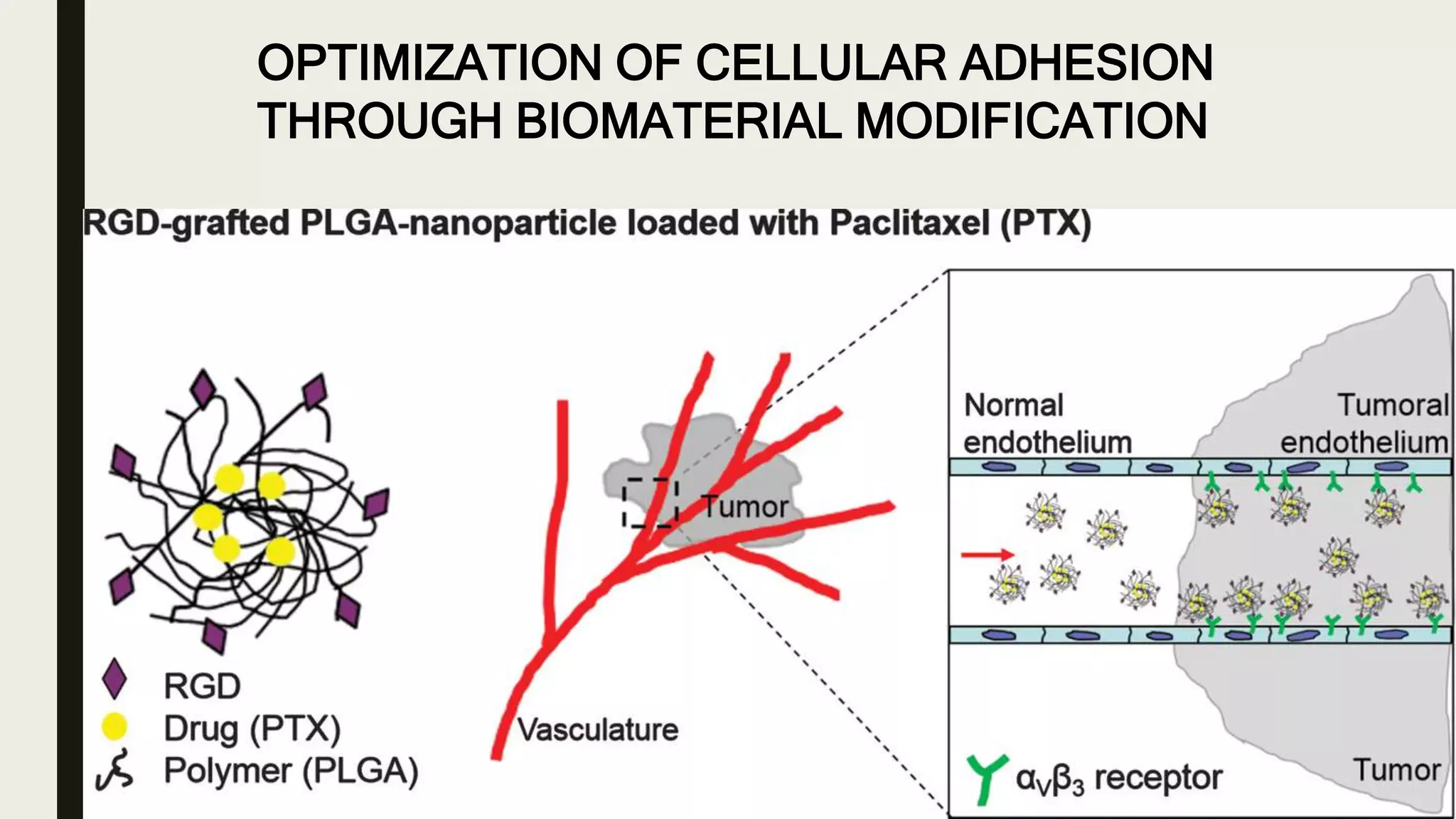
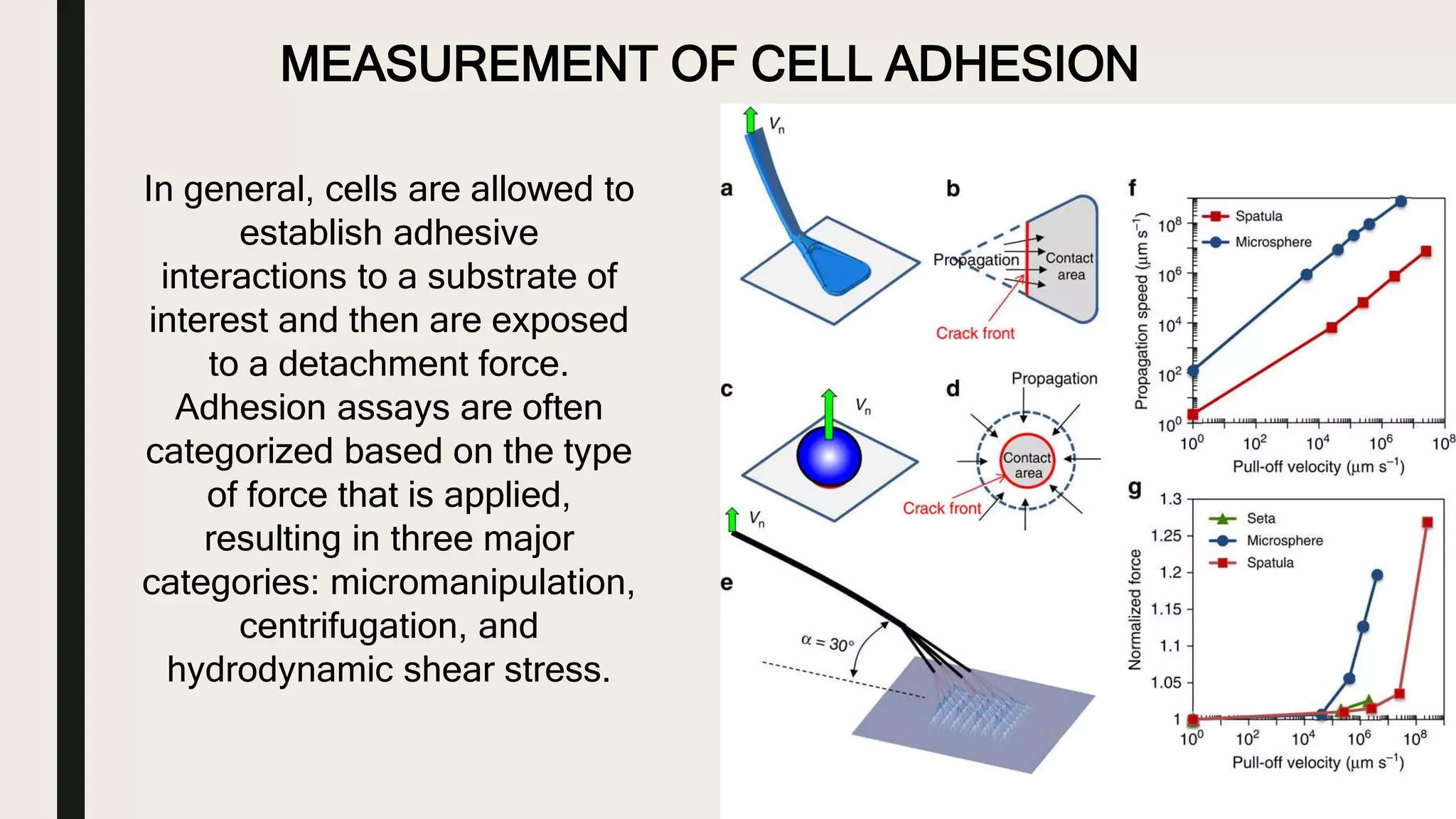
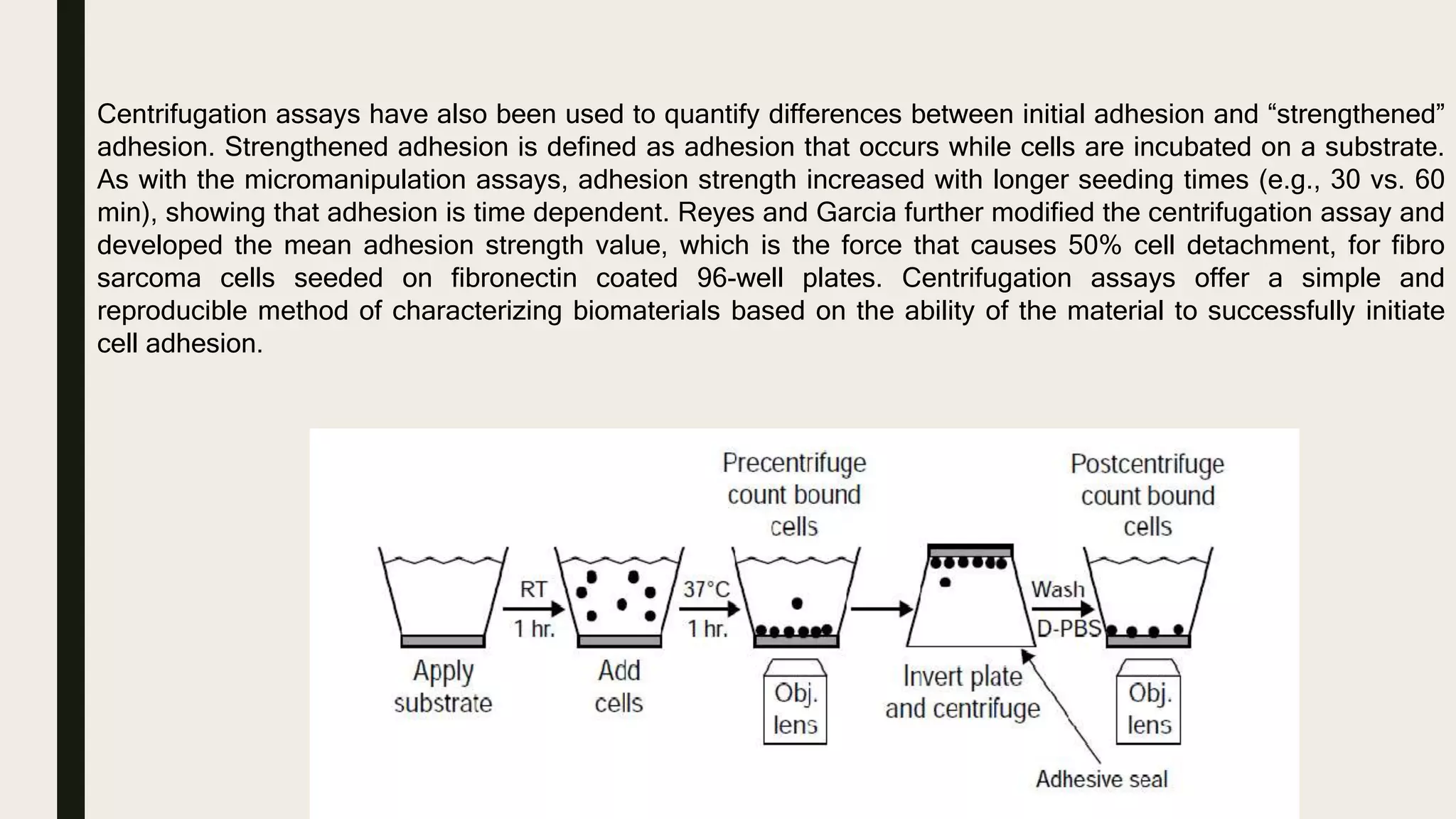
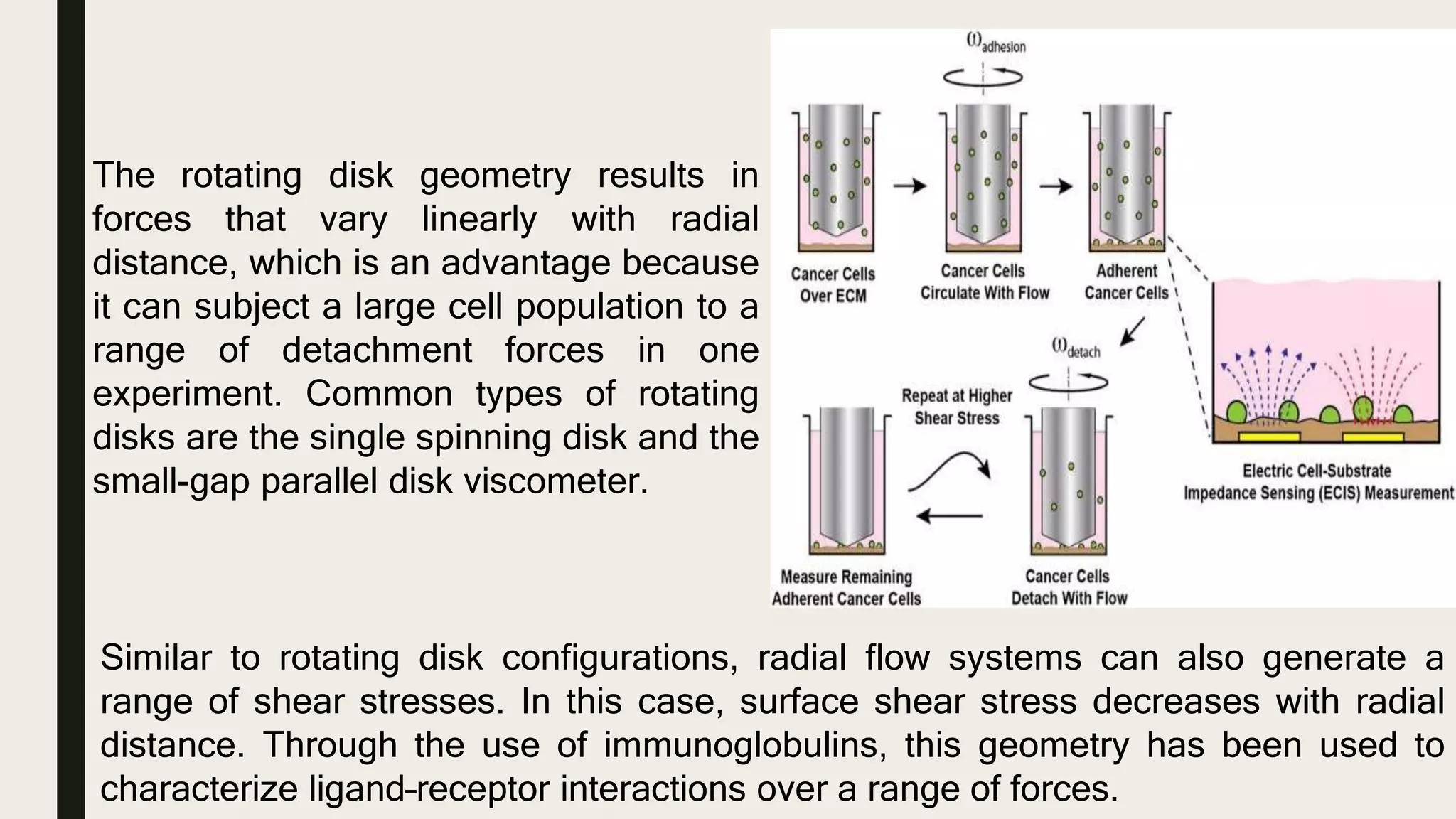
This document discusses characterization of adhesive interactions between cells and biomaterials. It begins by introducing how biomaterials are used to facilitate cell adhesion during tissue repair or replacement. It then discusses various adhesion receptors in native tissue like integrins, their classification and role in connecting the extracellular and intracellular environments. The document also covers optimization of cellular adhesion through biomaterial modification and different methods to measure cell adhesion like micromanipulation, centrifugation and applying hydrodynamic shear stress. It concludes that modifying biomaterials to mimic native adhesive interactions can help control downstream cell responses and have therapeutic applications.