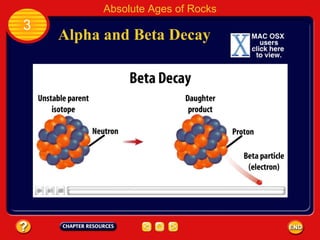
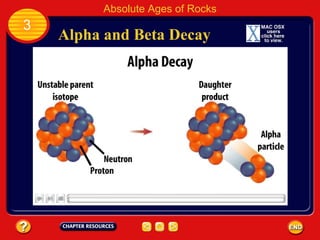
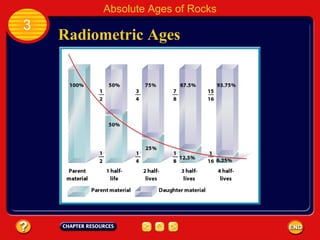
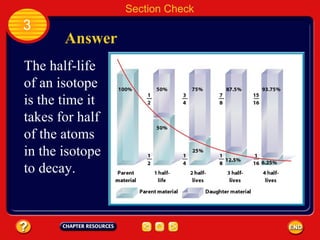
Absolute age refers to the precise age of a rock in years, which geologists determine through radioactive dating techniques. Certain isotopes are radioactive and decay over time at a fixed rate called their half-life. By measuring the ratio of the original, or parent, isotope to the decayed daughter product in a rock and knowing the half-life, geologists can calculate the absolute age. Radiometric dating has allowed scientists to determine that the oldest rocks on Earth are approximately 3.96 billion years old, and that the Earth itself formed about 4.5 billion years ago.