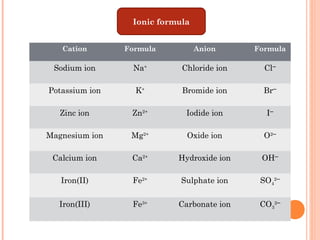
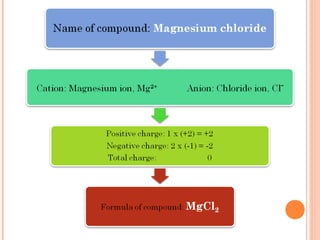
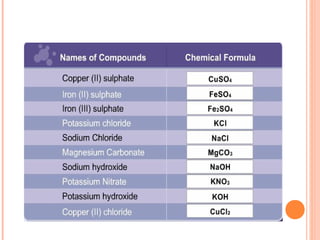
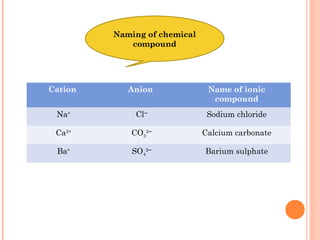
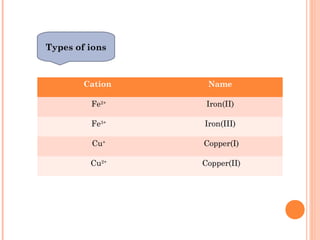
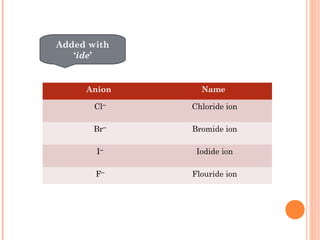
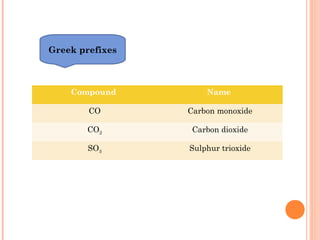
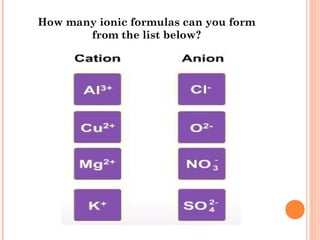
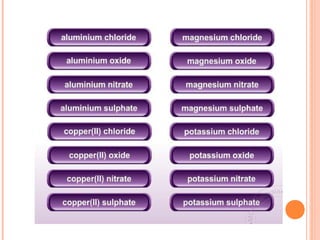
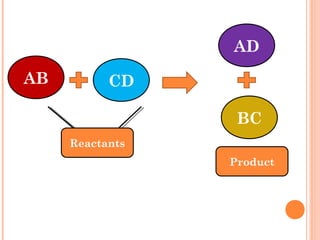
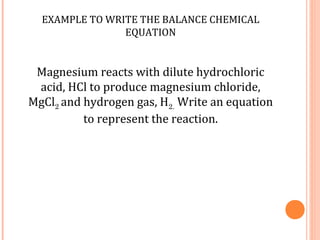
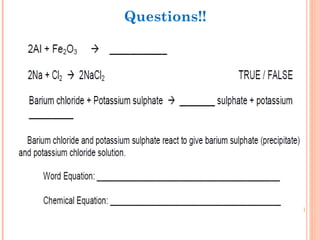
The document discusses chemical formulas and equations. It provides tables listing common cations and anions along with their formulas. Examples of naming ionic compounds based on their constituent ions are given. Greek prefixes in chemical names are explained. The document introduces writing and balancing chemical equations to represent chemical reactions.