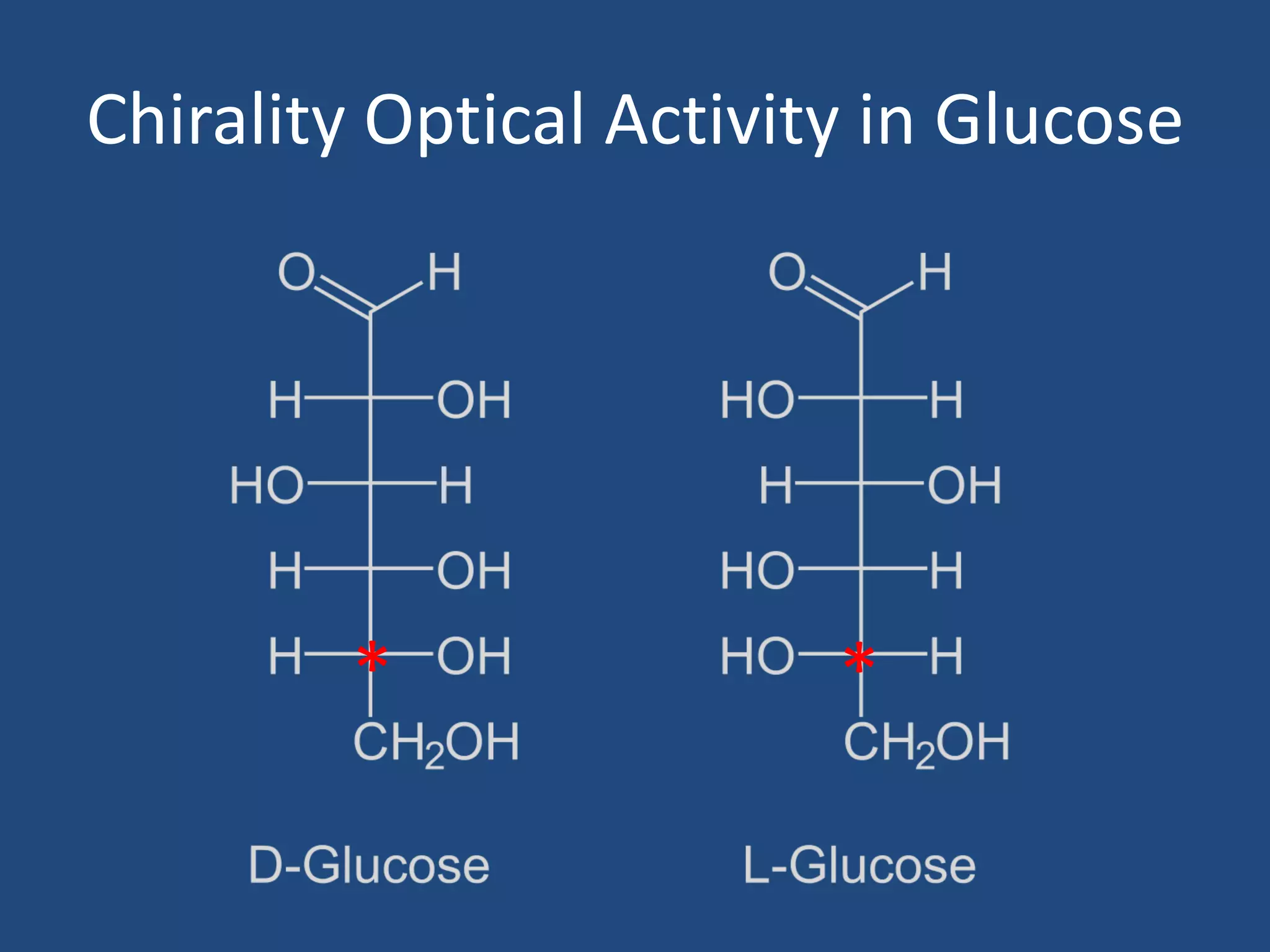
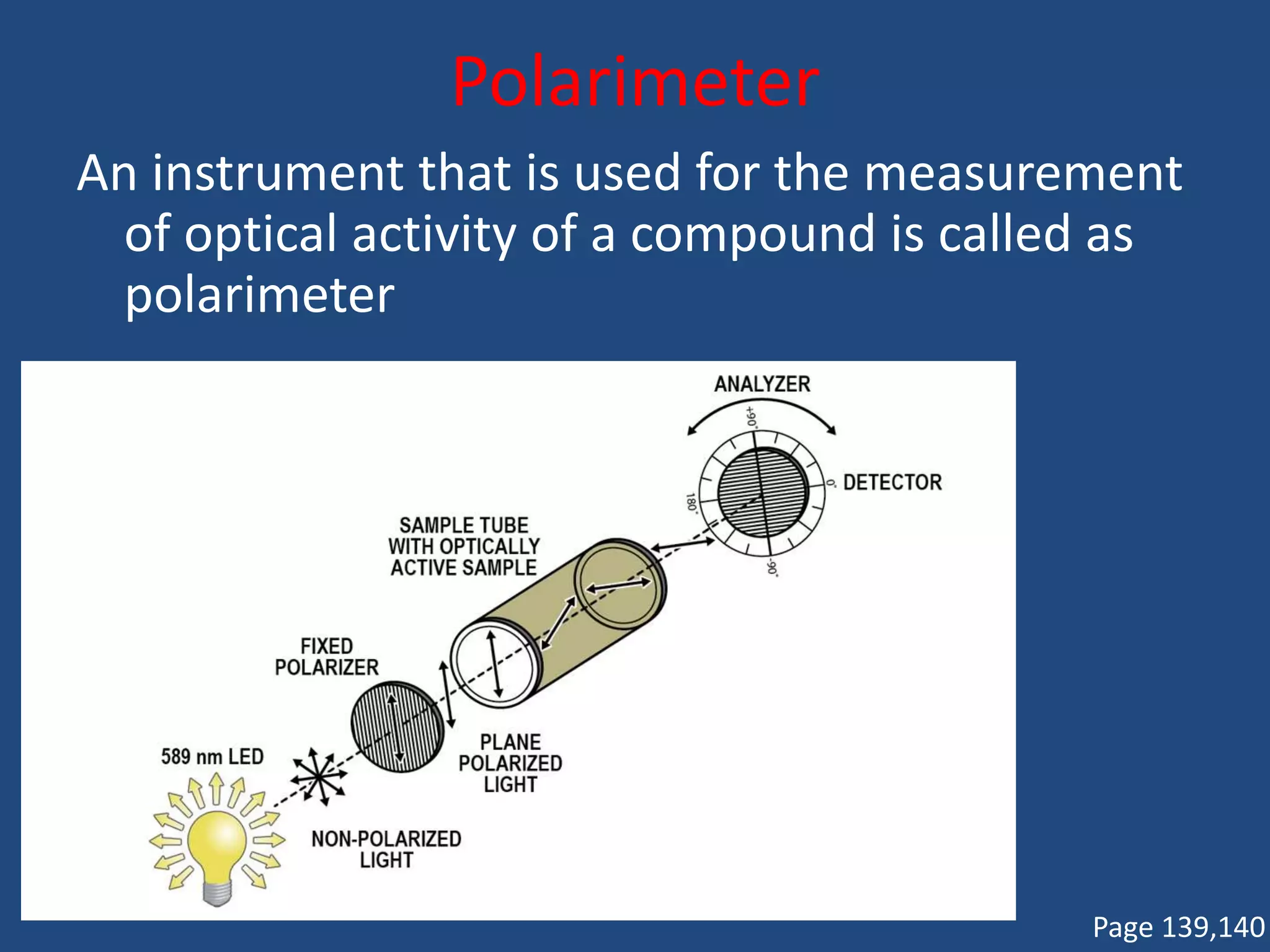
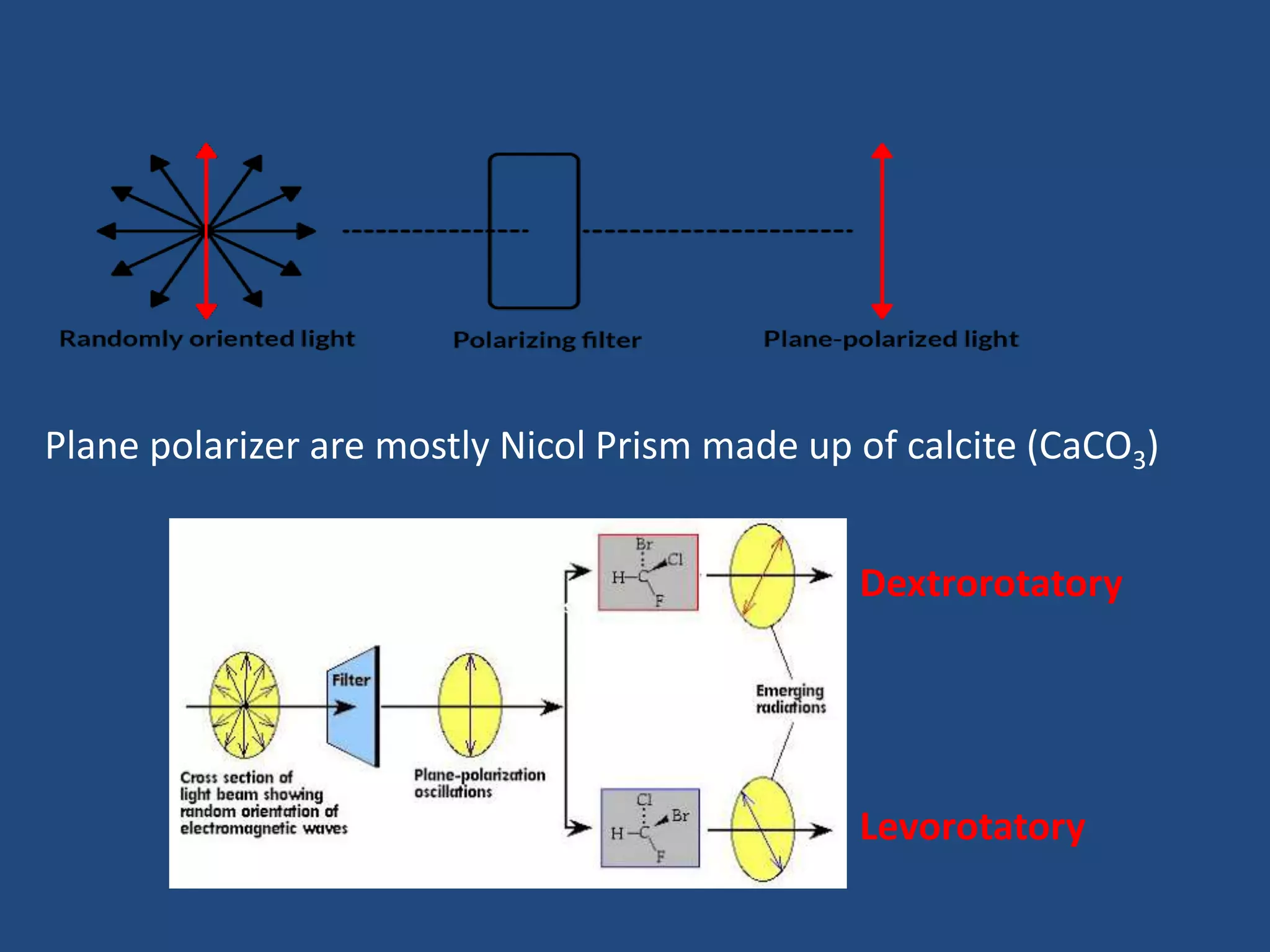
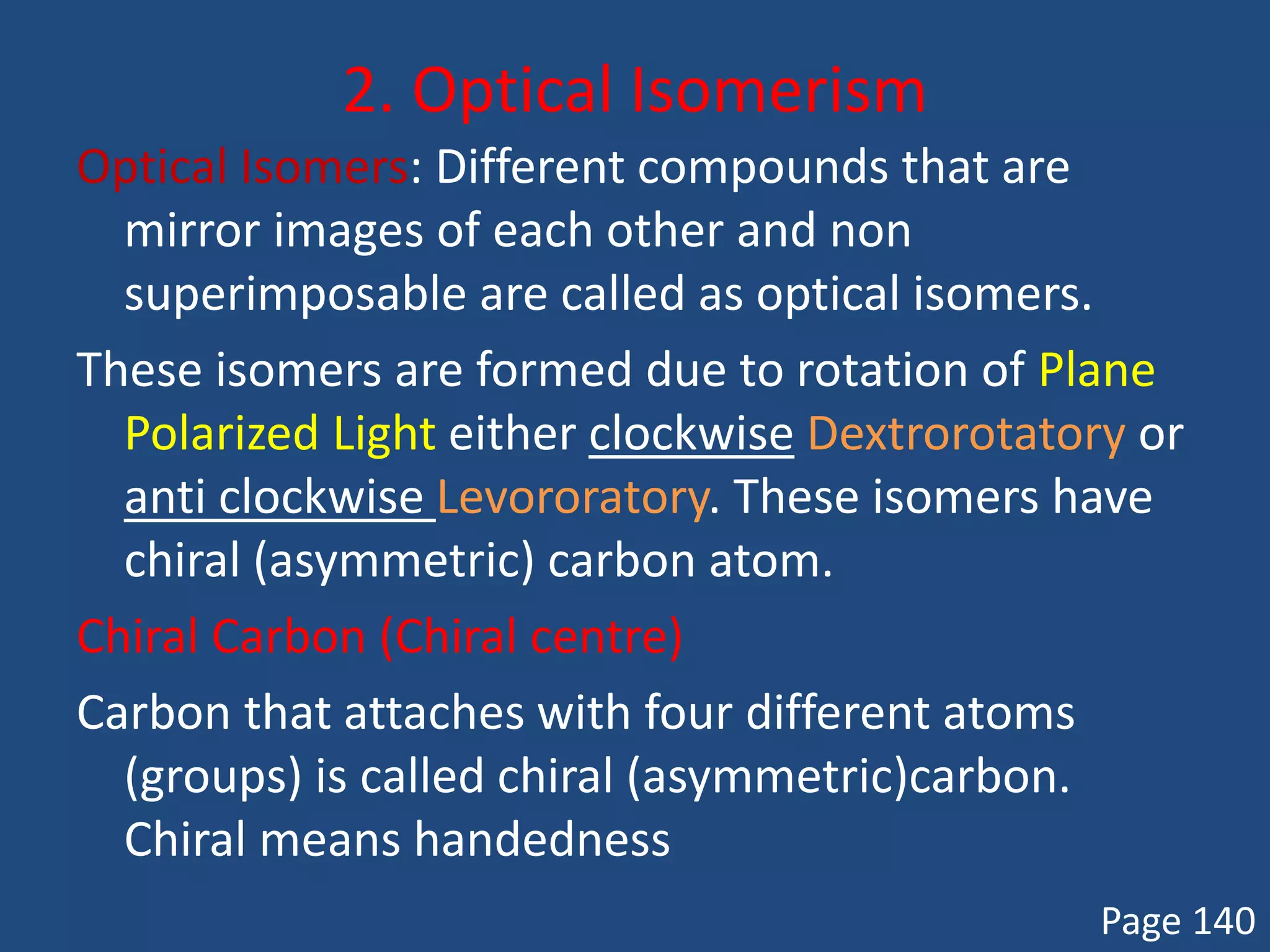
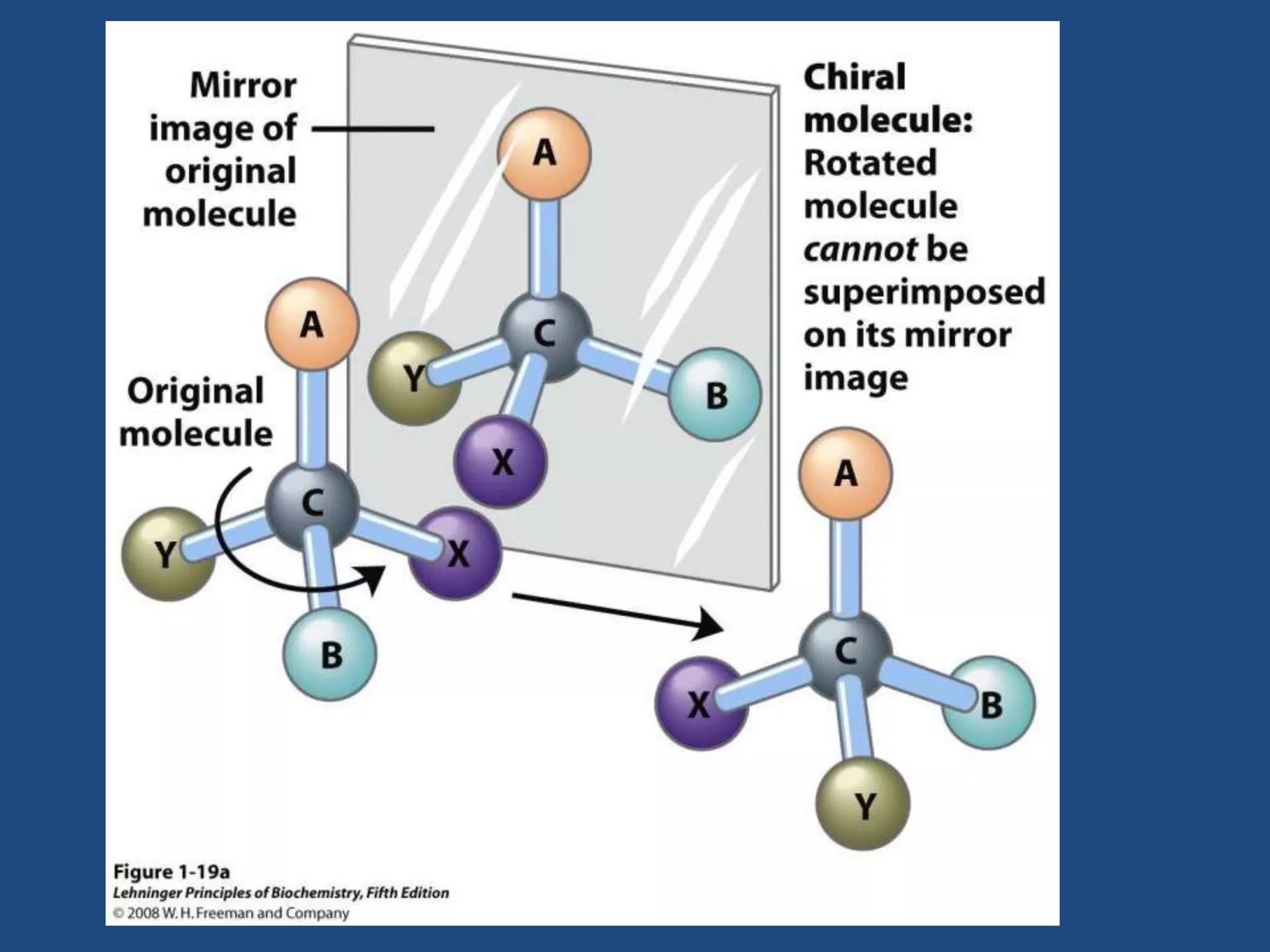
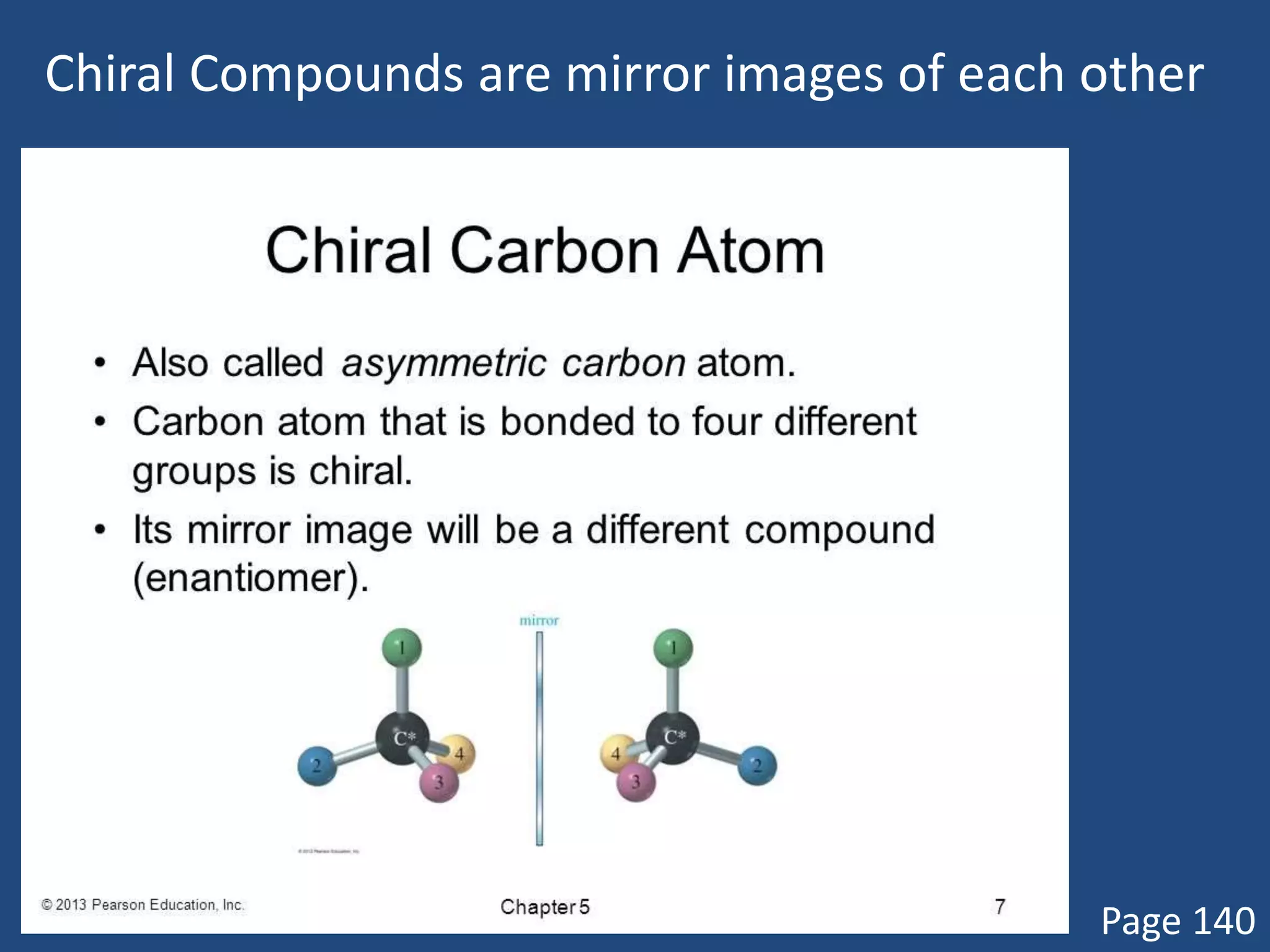
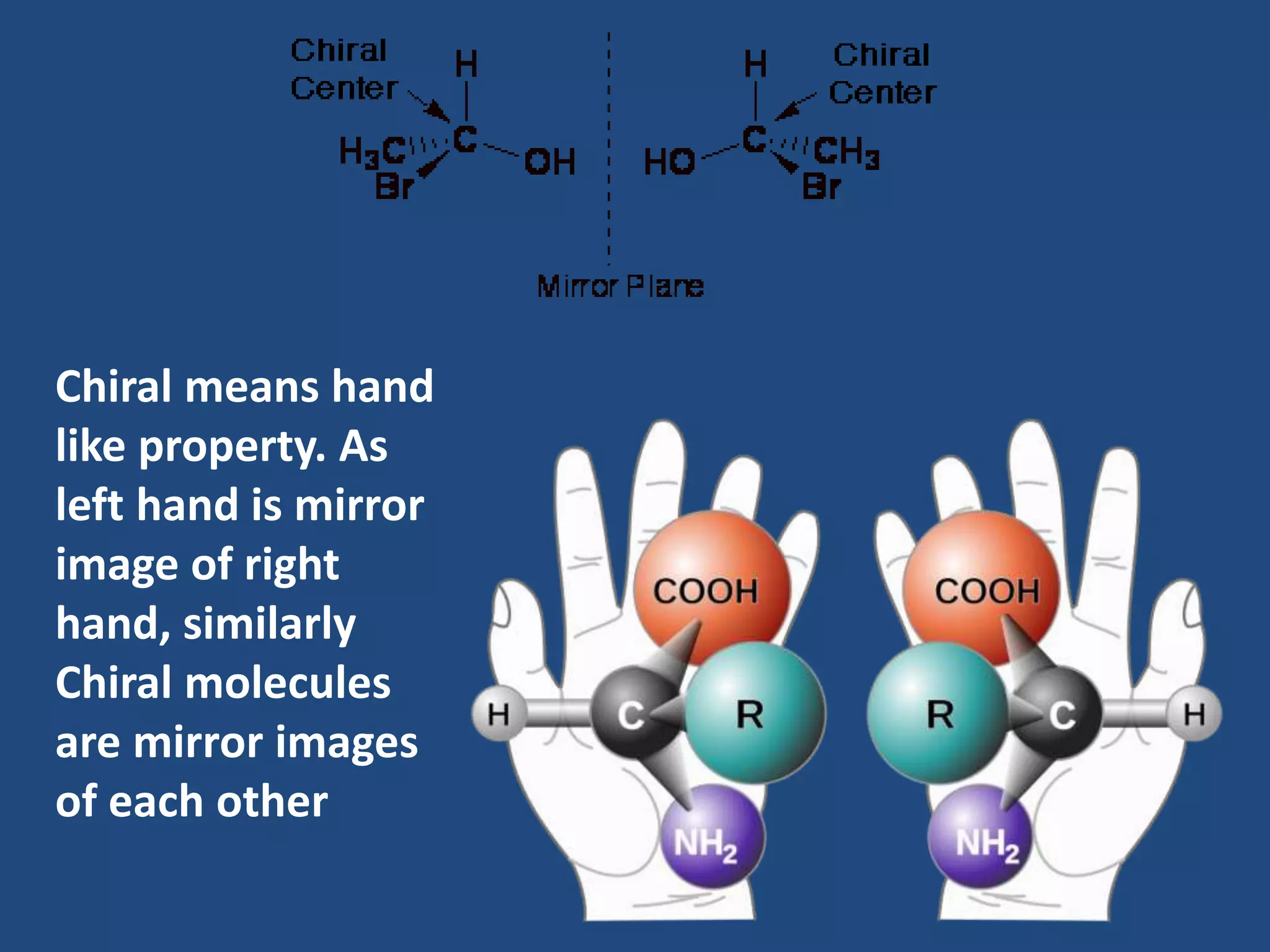
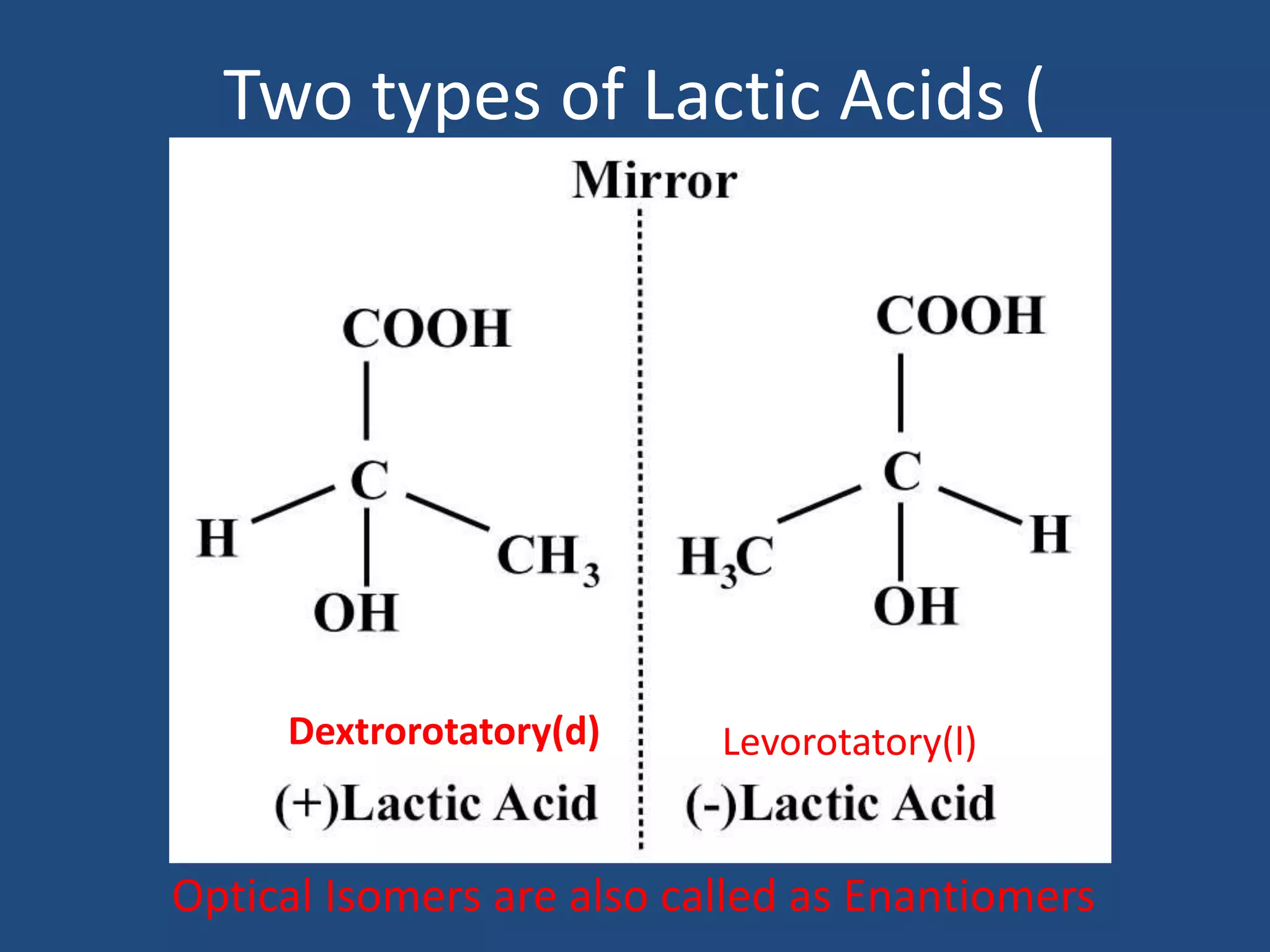
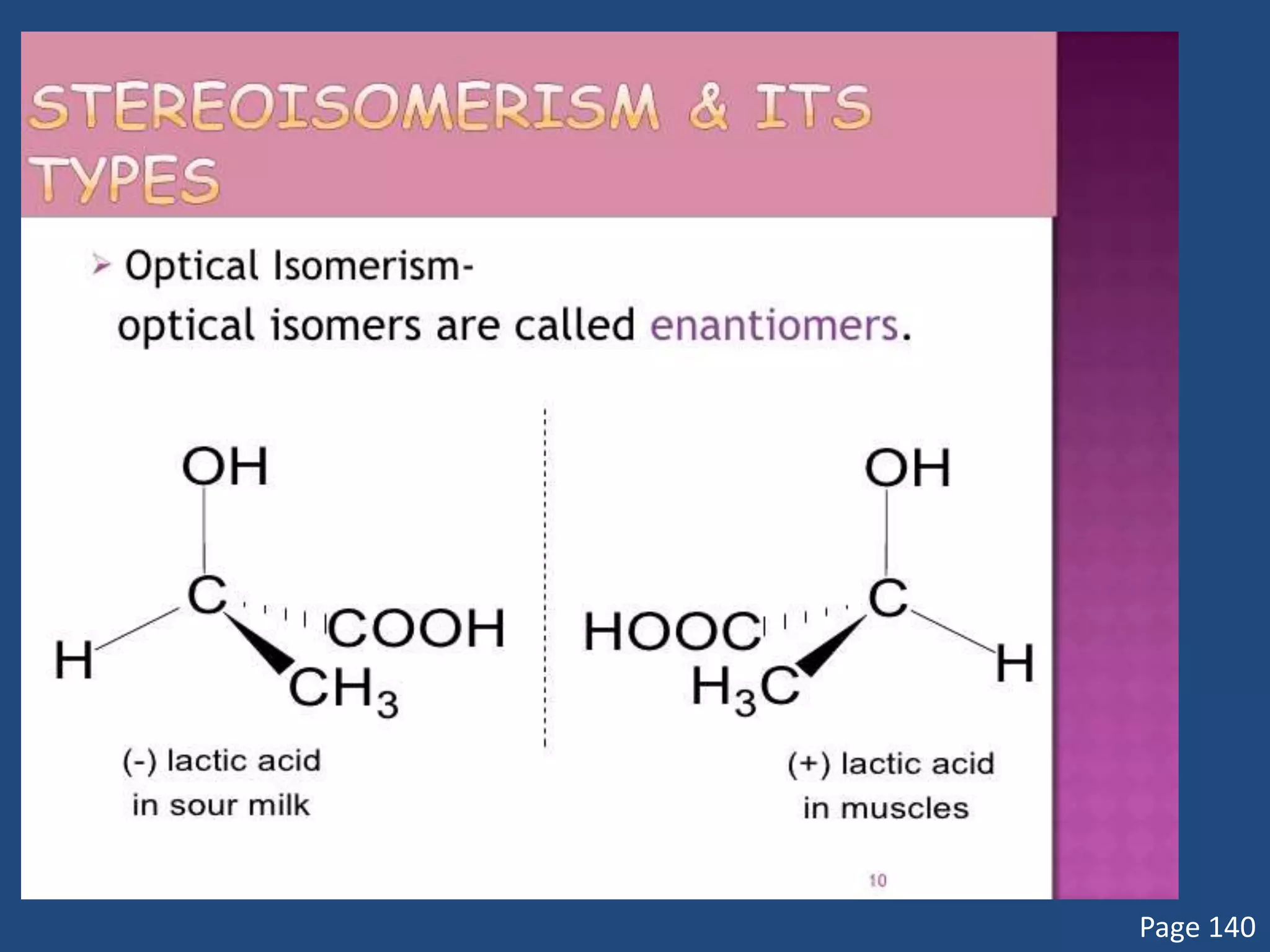
When a compound rotates plane polarized light, it is said to be optically active. There are two types of optical isomers: dextrorotatory isomers rotate polarized light to the right, while levorotatory isomers rotate it to the left. Optical isomers are non-superimposable mirror images of each other that contain a chiral or asymmetric carbon atom bonded to four different groups. A polarimeter is used to measure the optical activity of compounds.