This document provides an overview of chemistry concepts including:
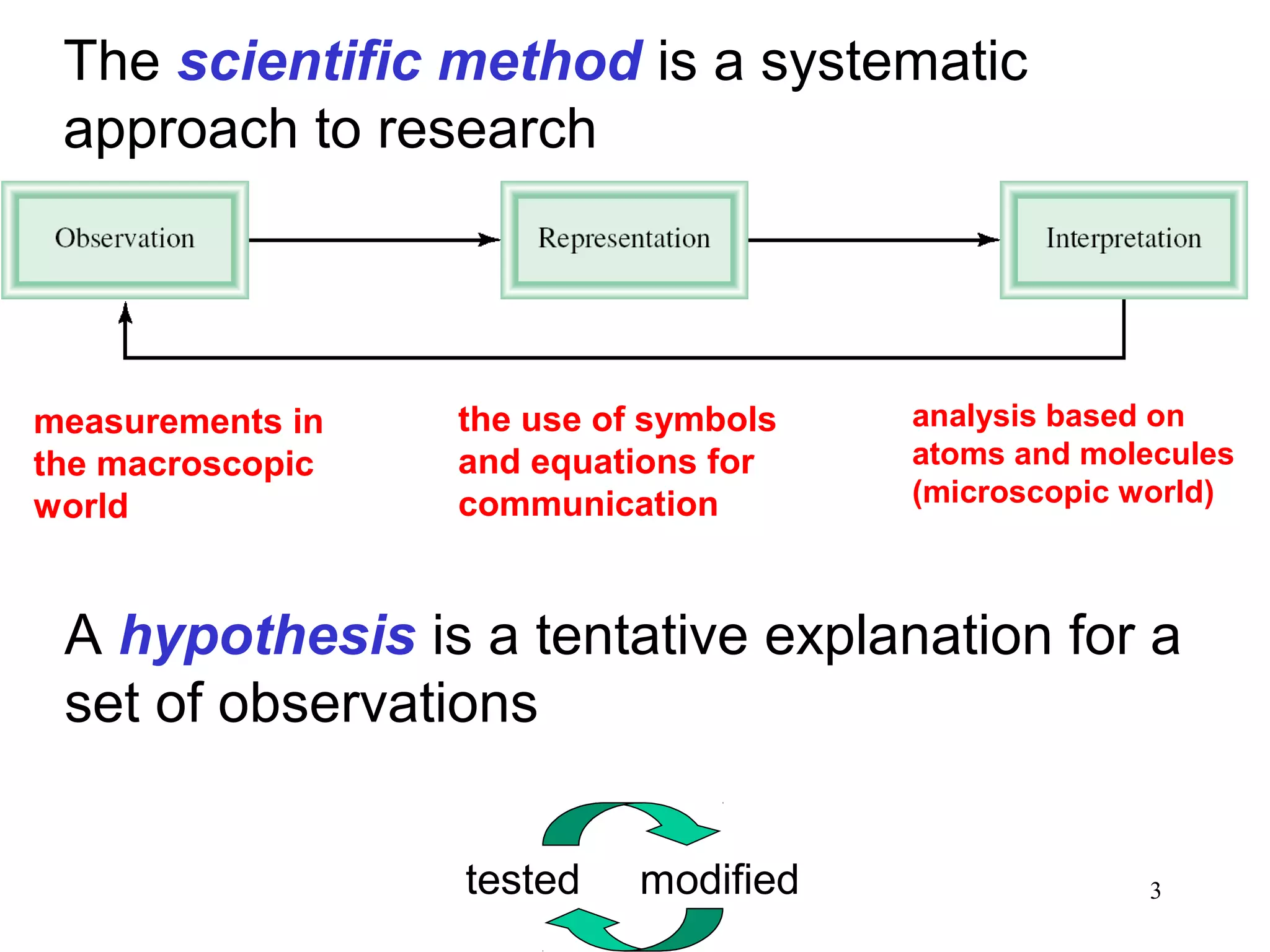
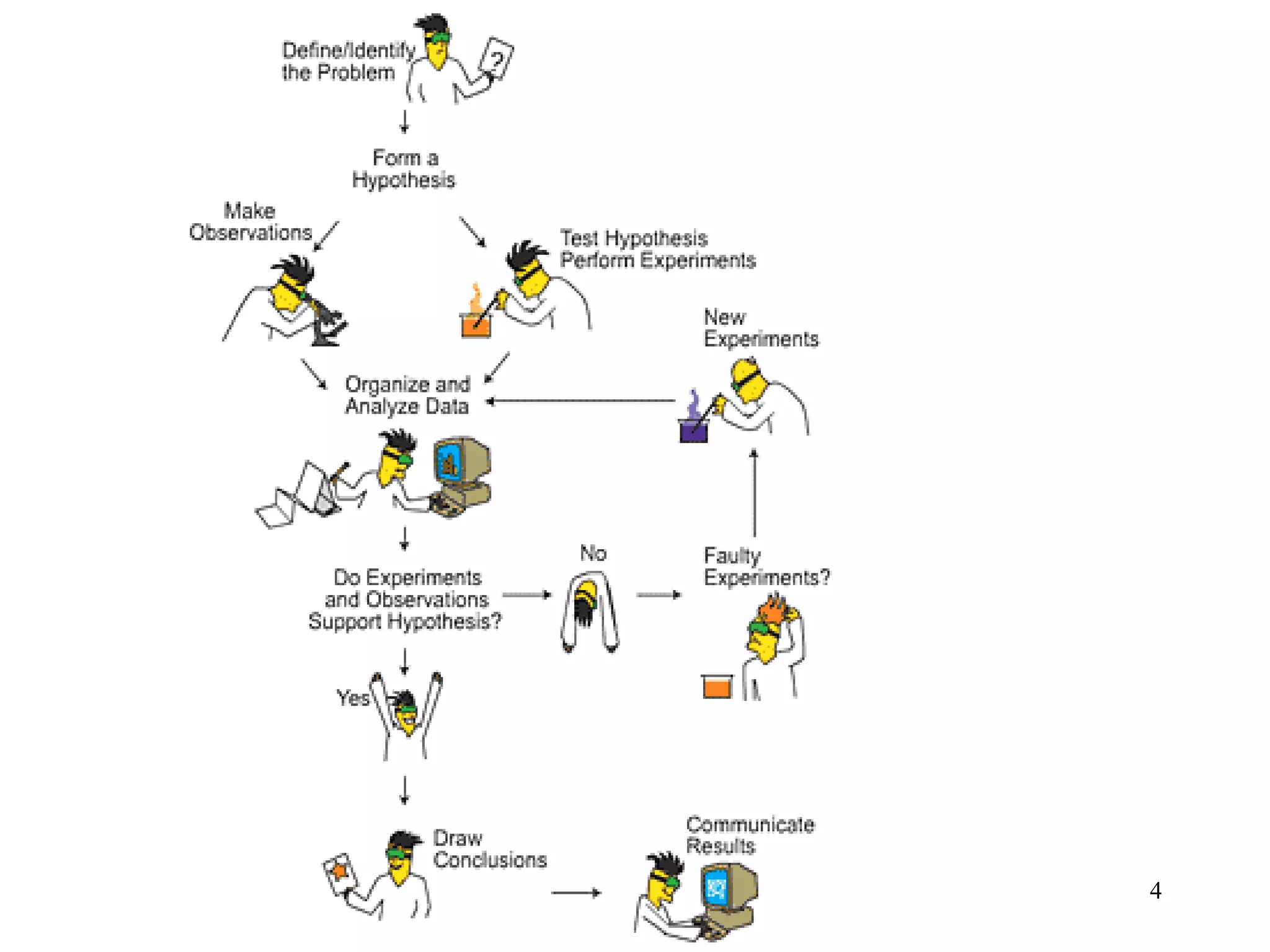
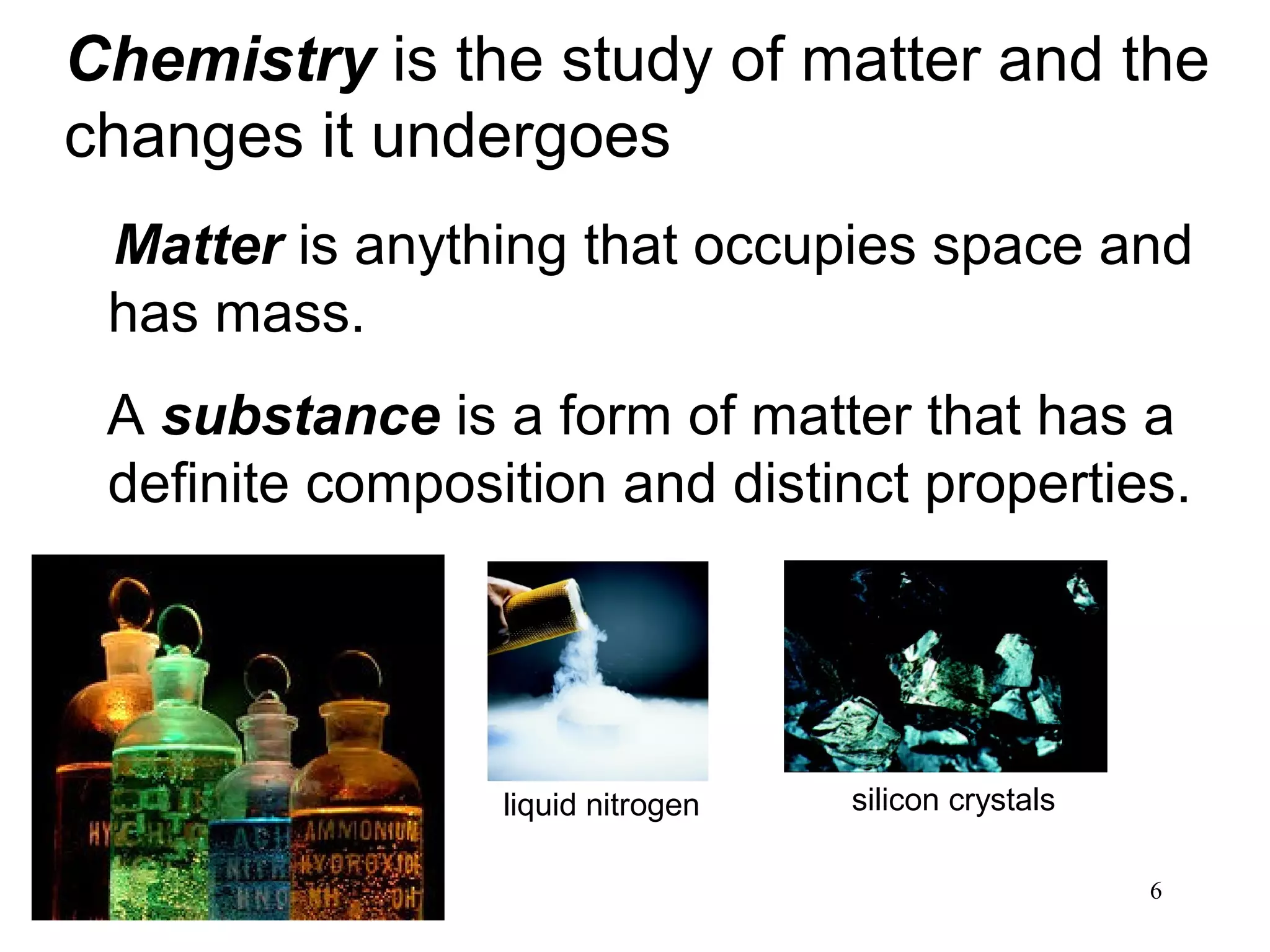
1. Chemistry is the study of matter and the changes it undergoes. The scientific method uses a systematic approach involving hypotheses, experiments, and analysis.
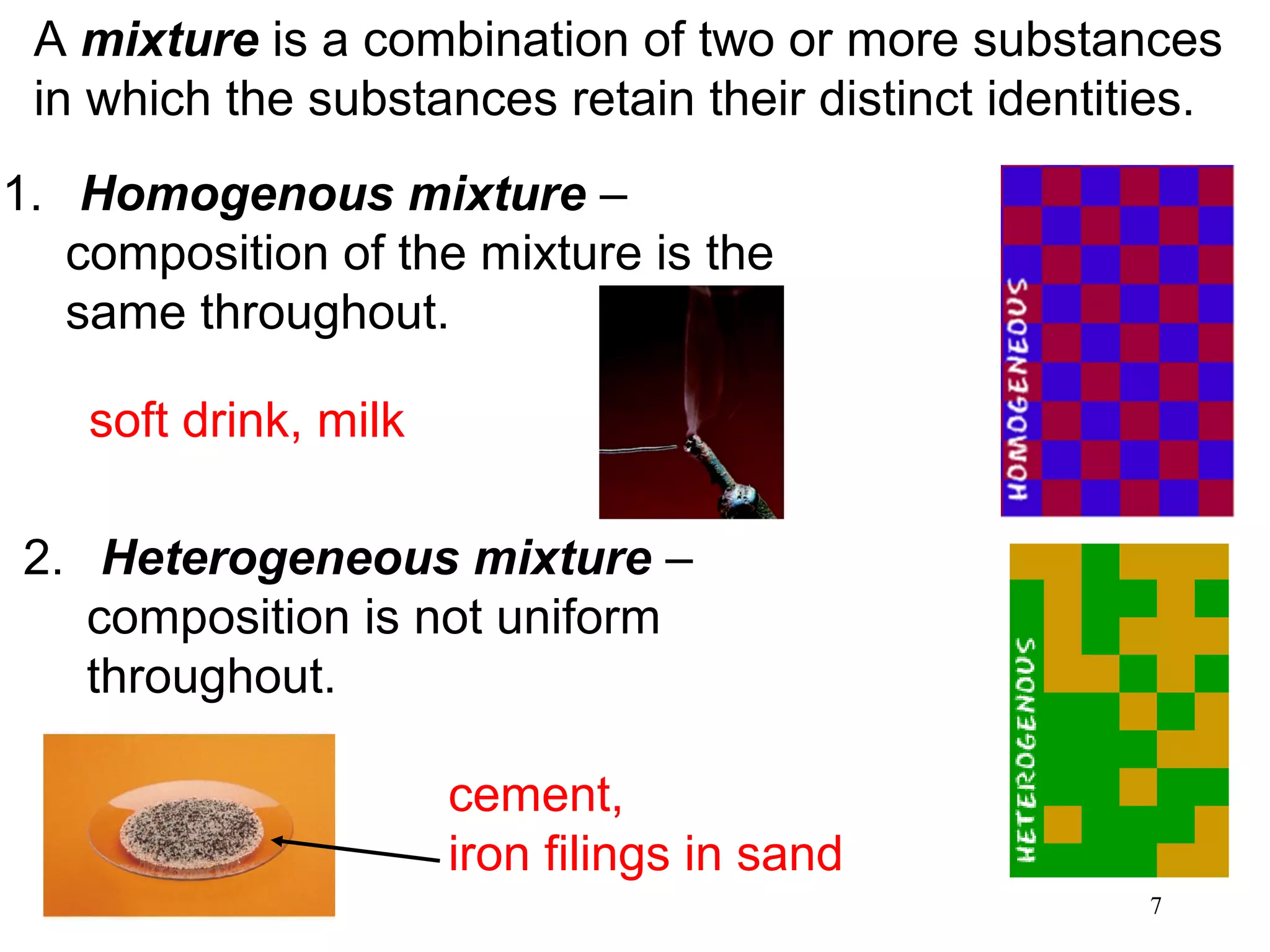
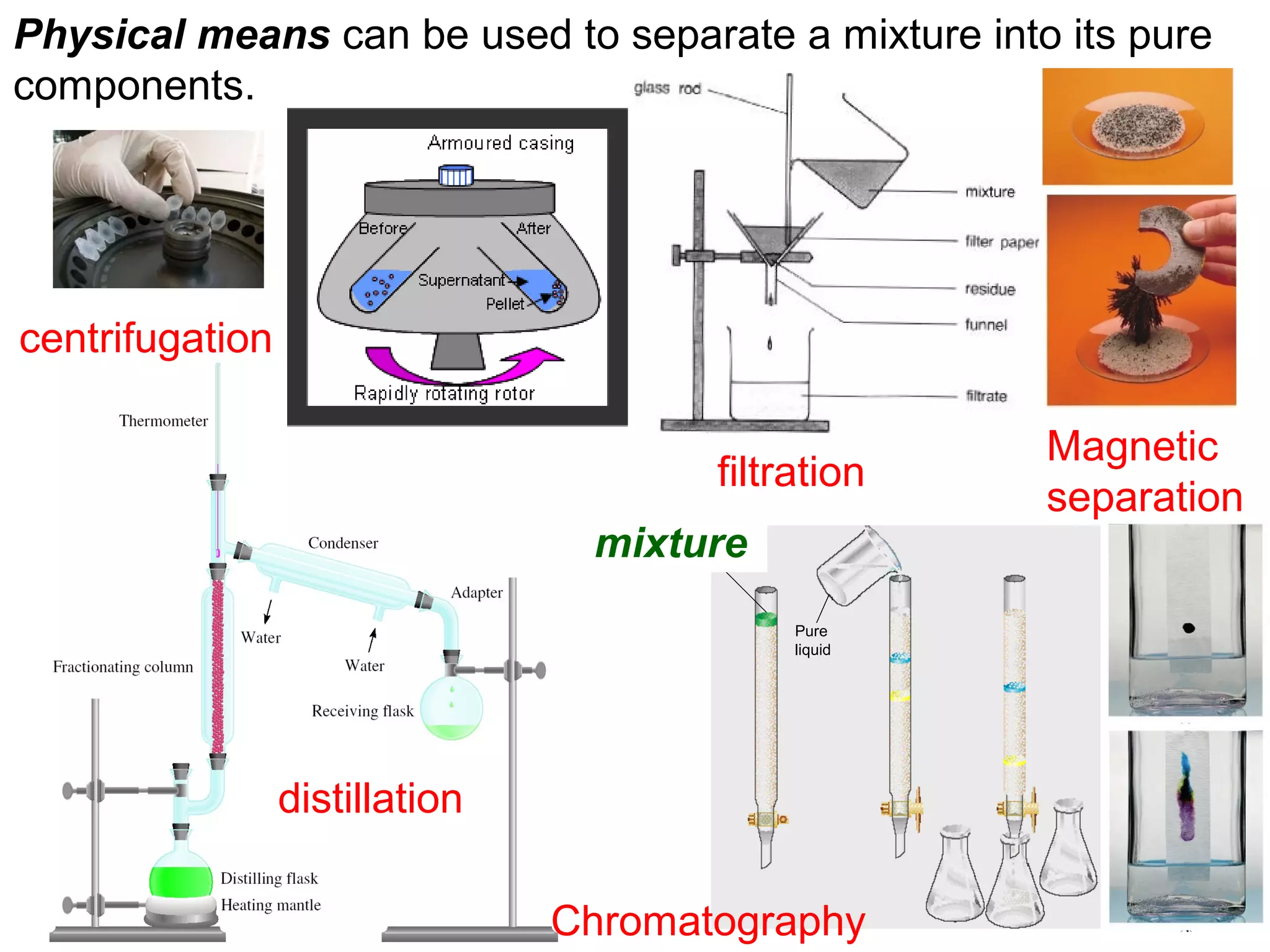
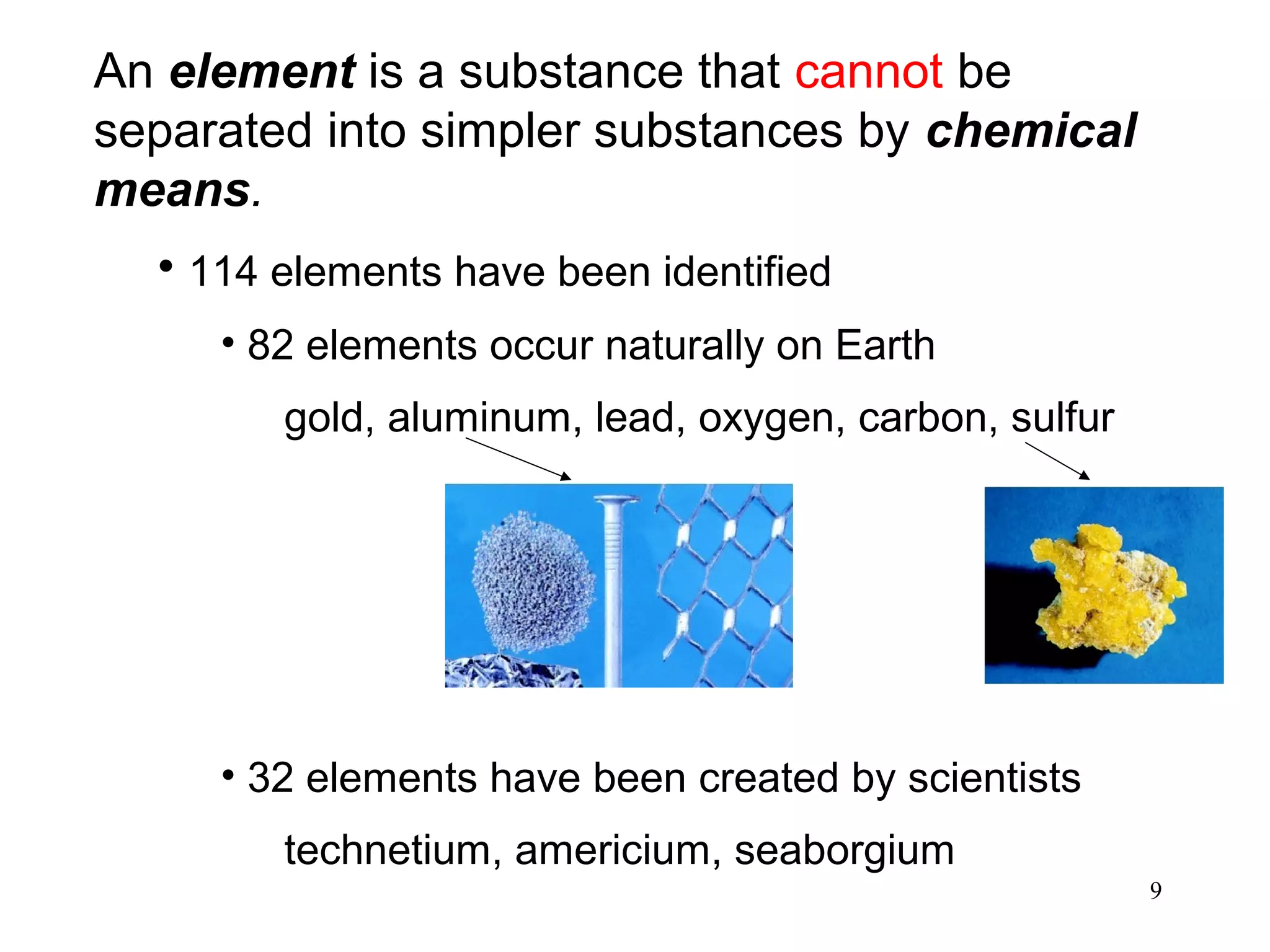
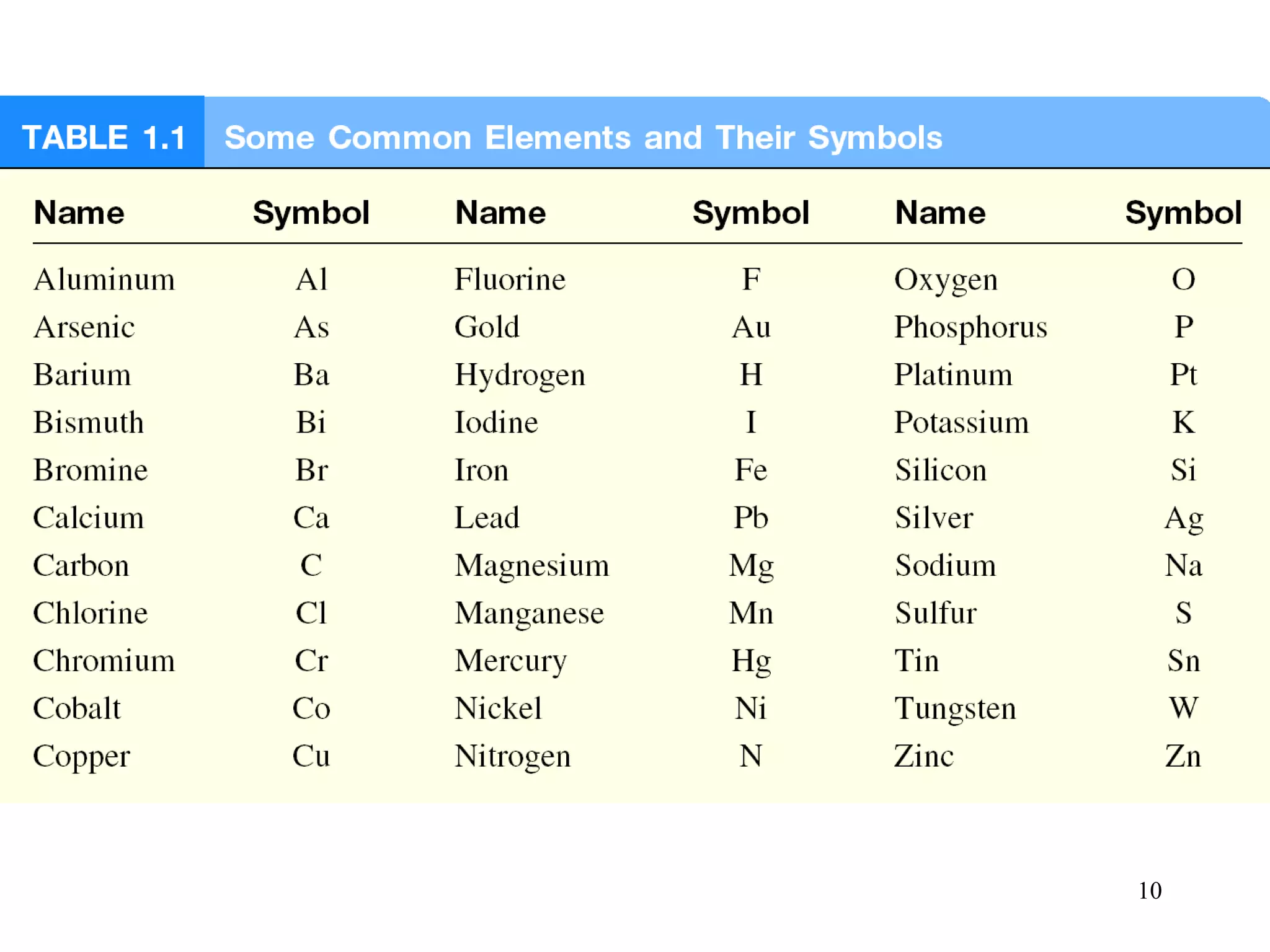
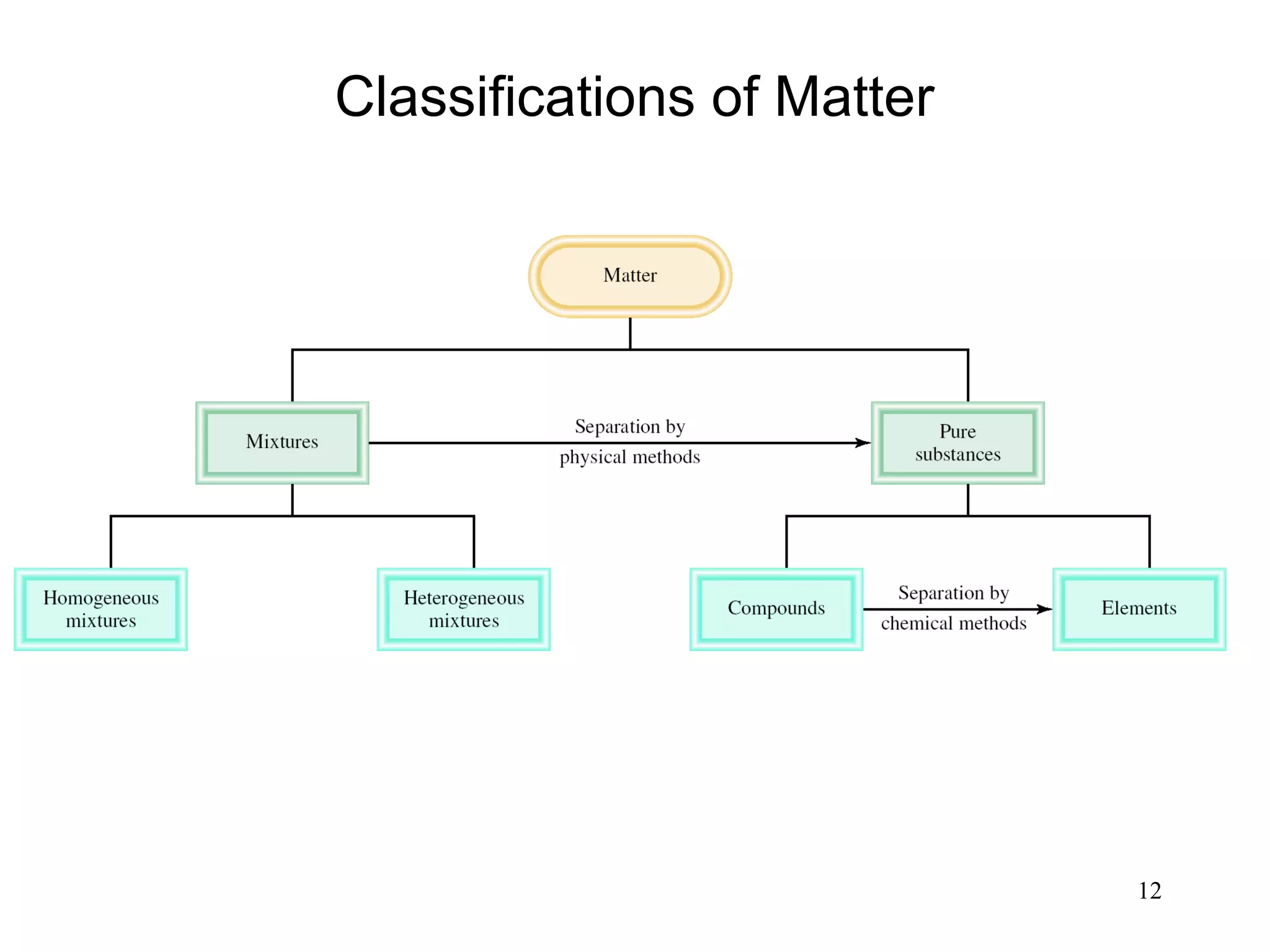
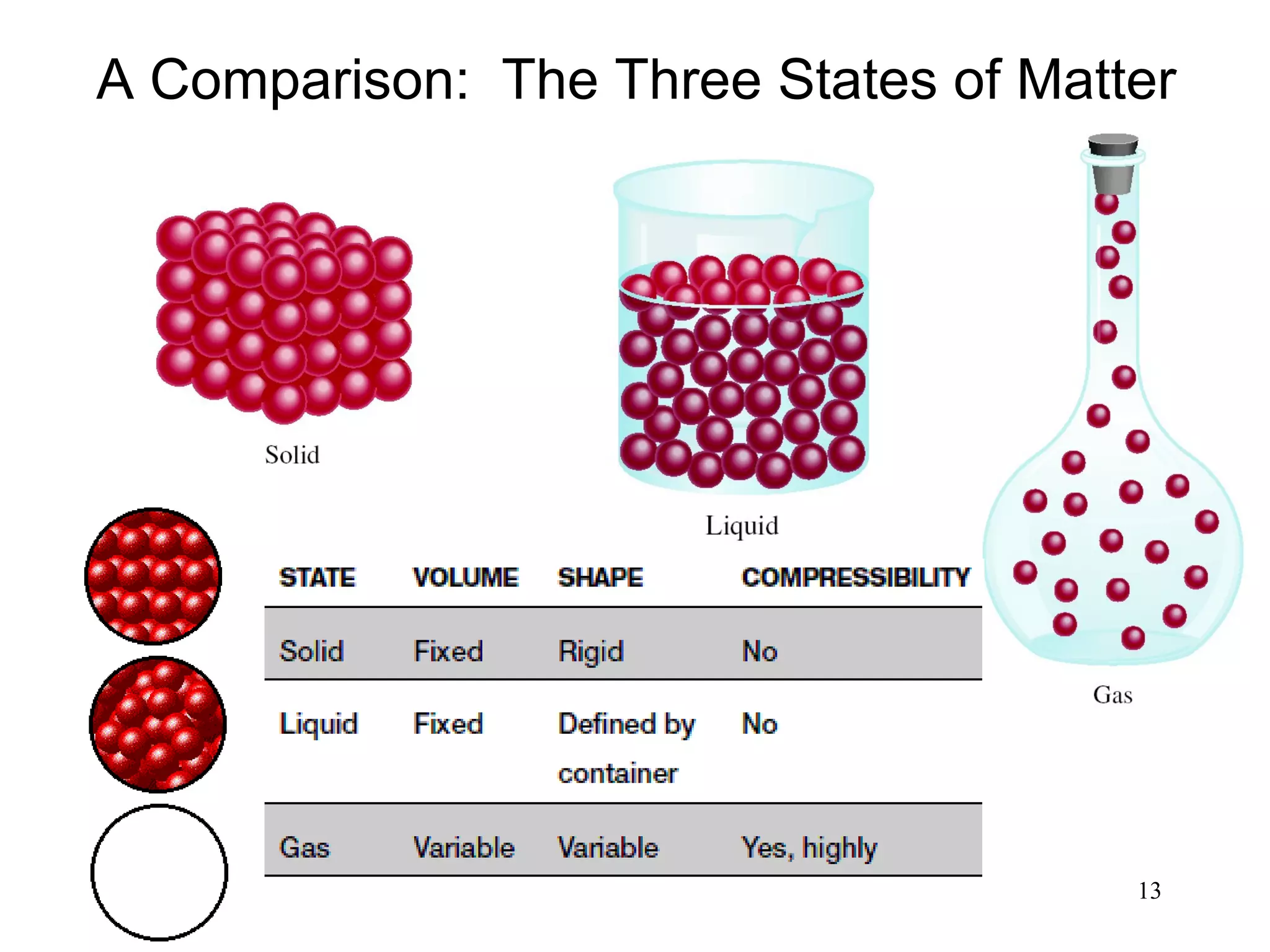
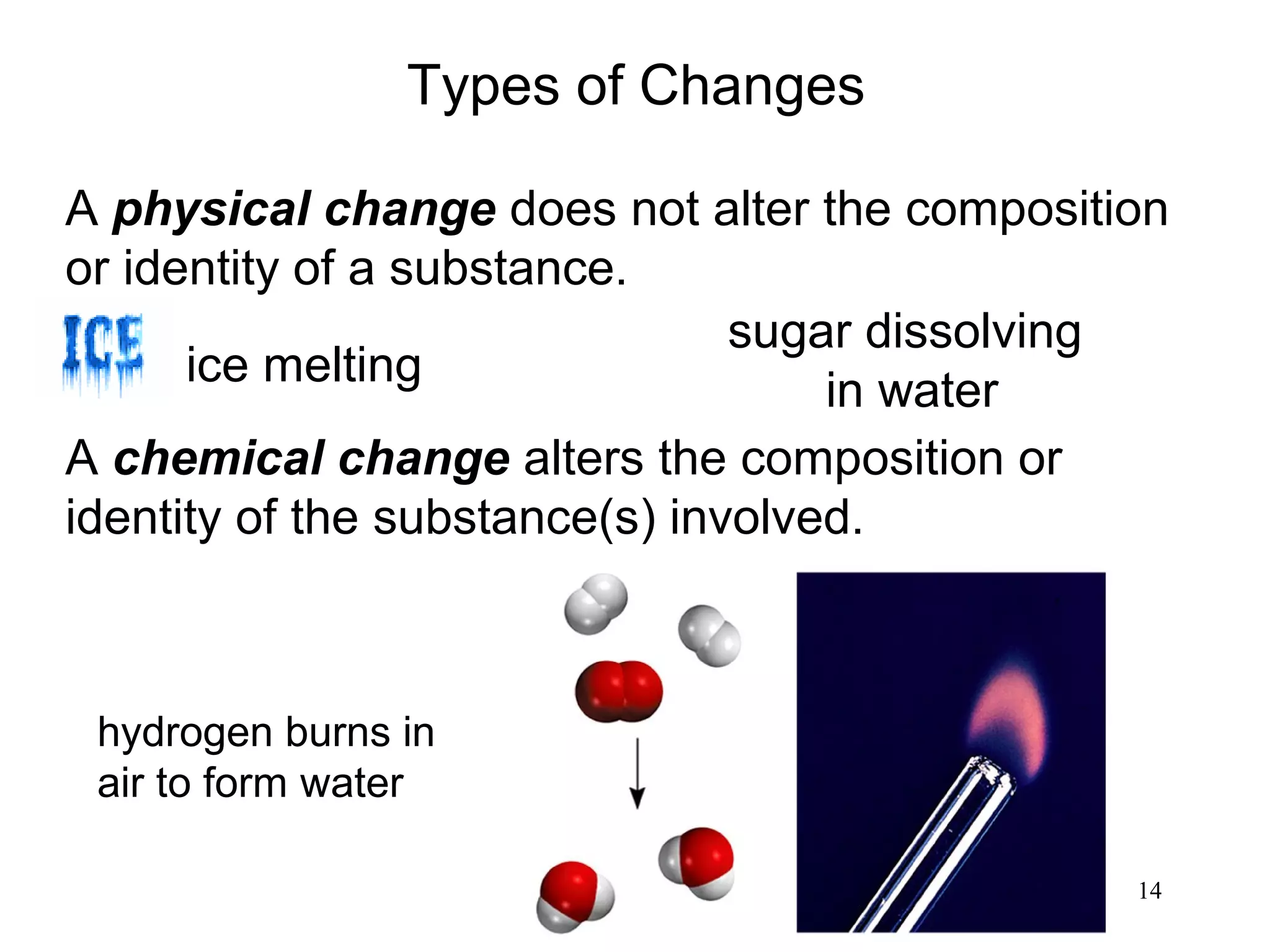
2. Matter can exist as elements, compounds, mixtures, and in three main states - solids, liquids, and gases. Chemical and physical changes alter substances in different ways.
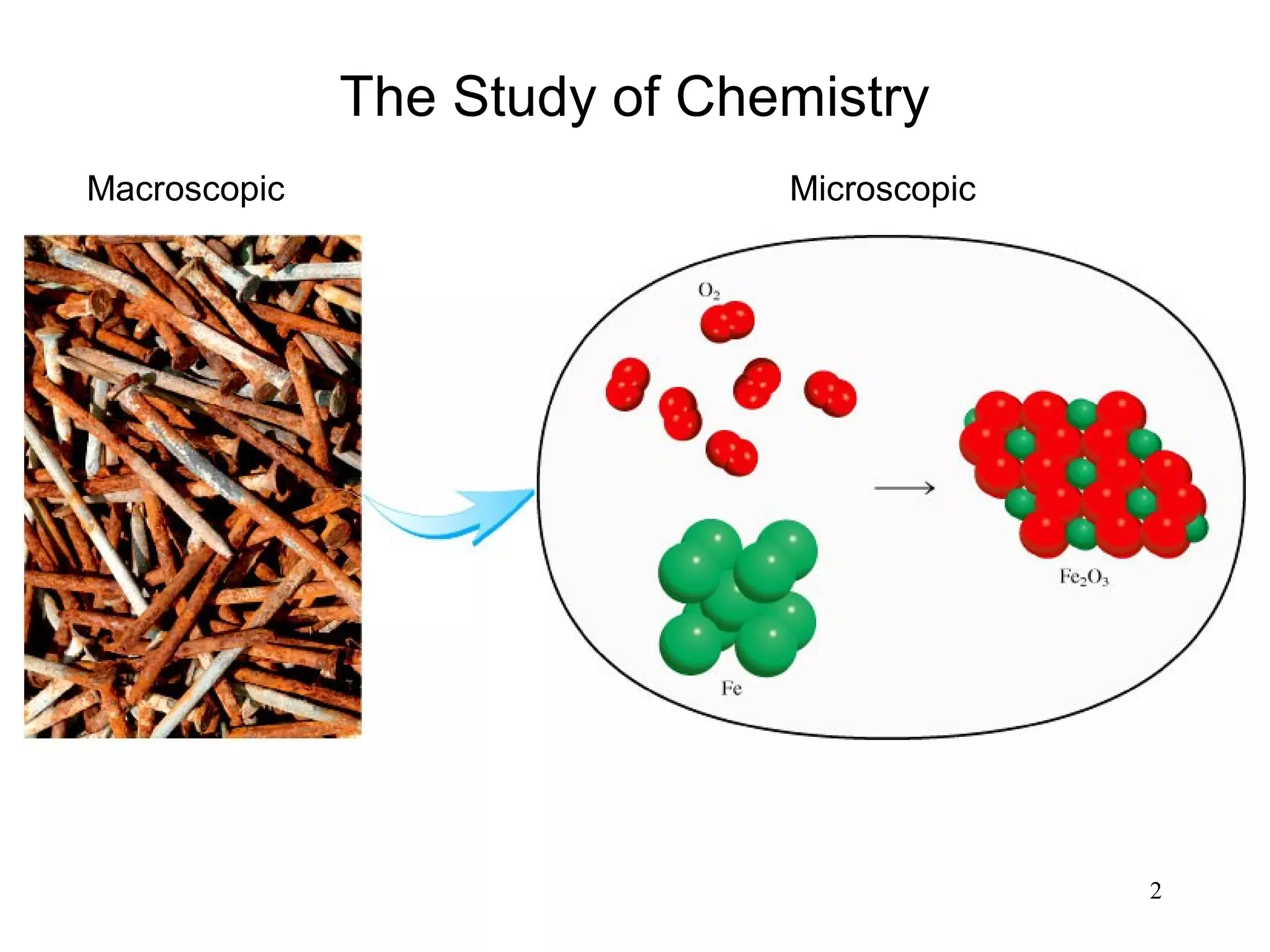
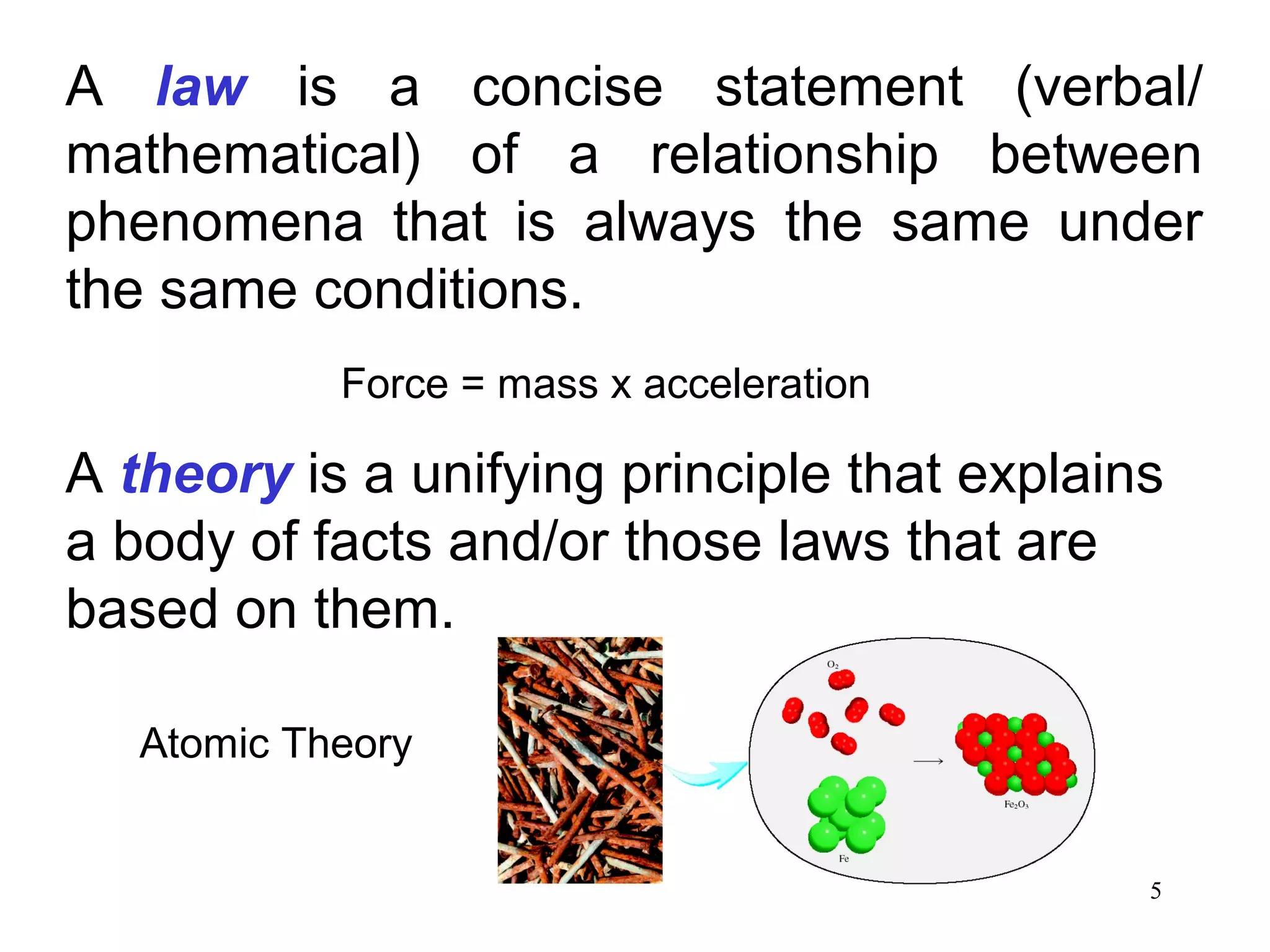
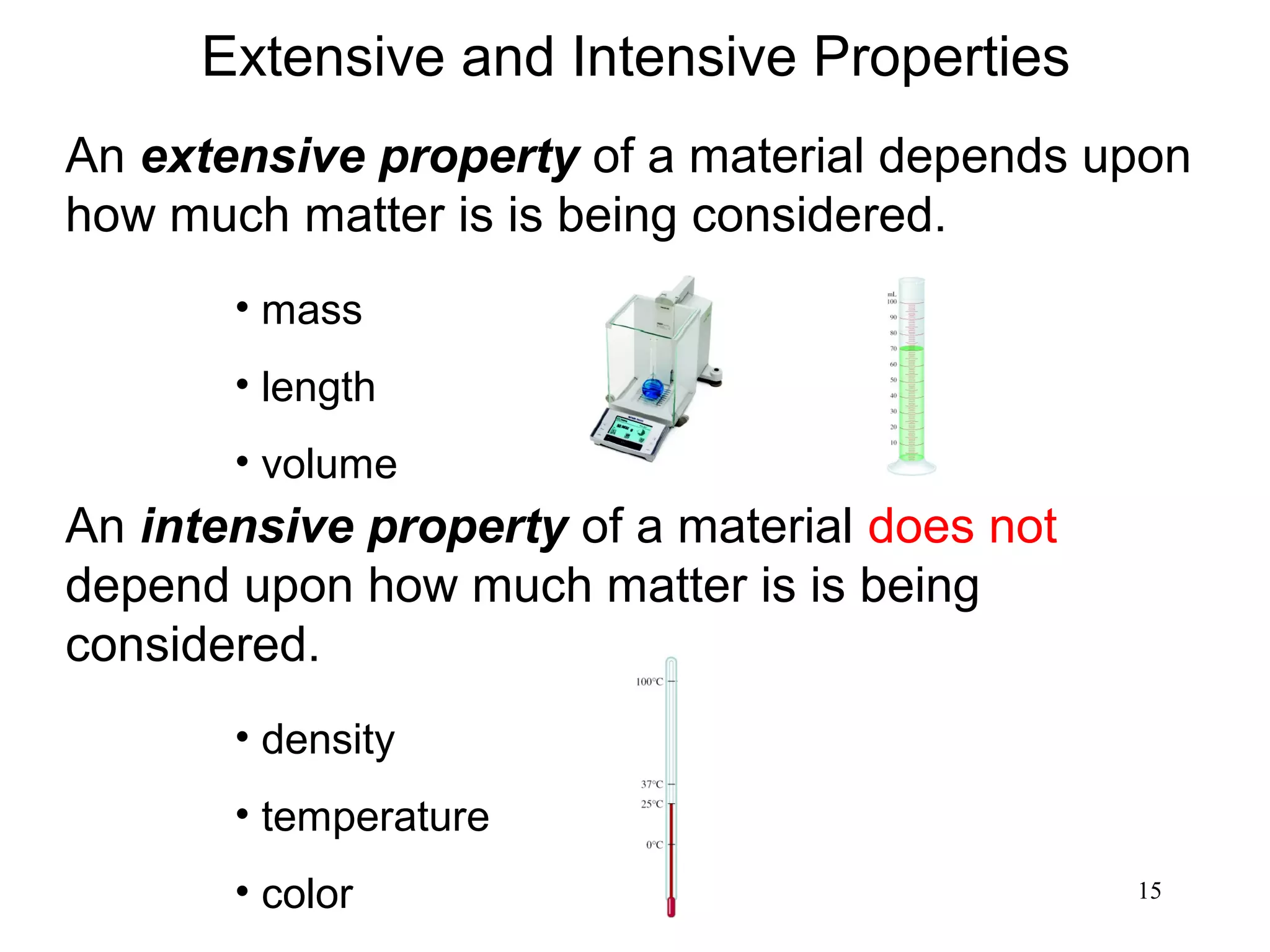
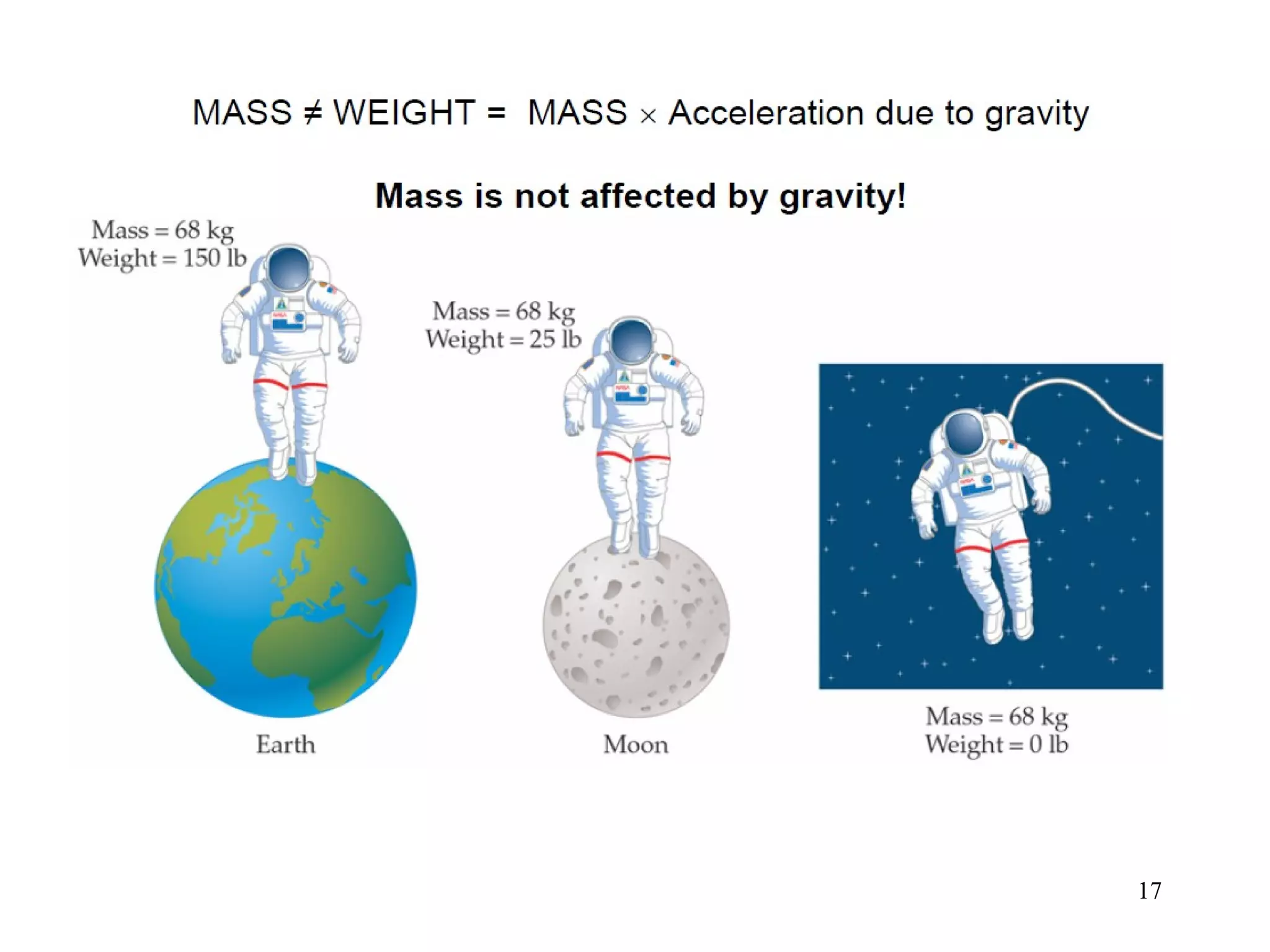
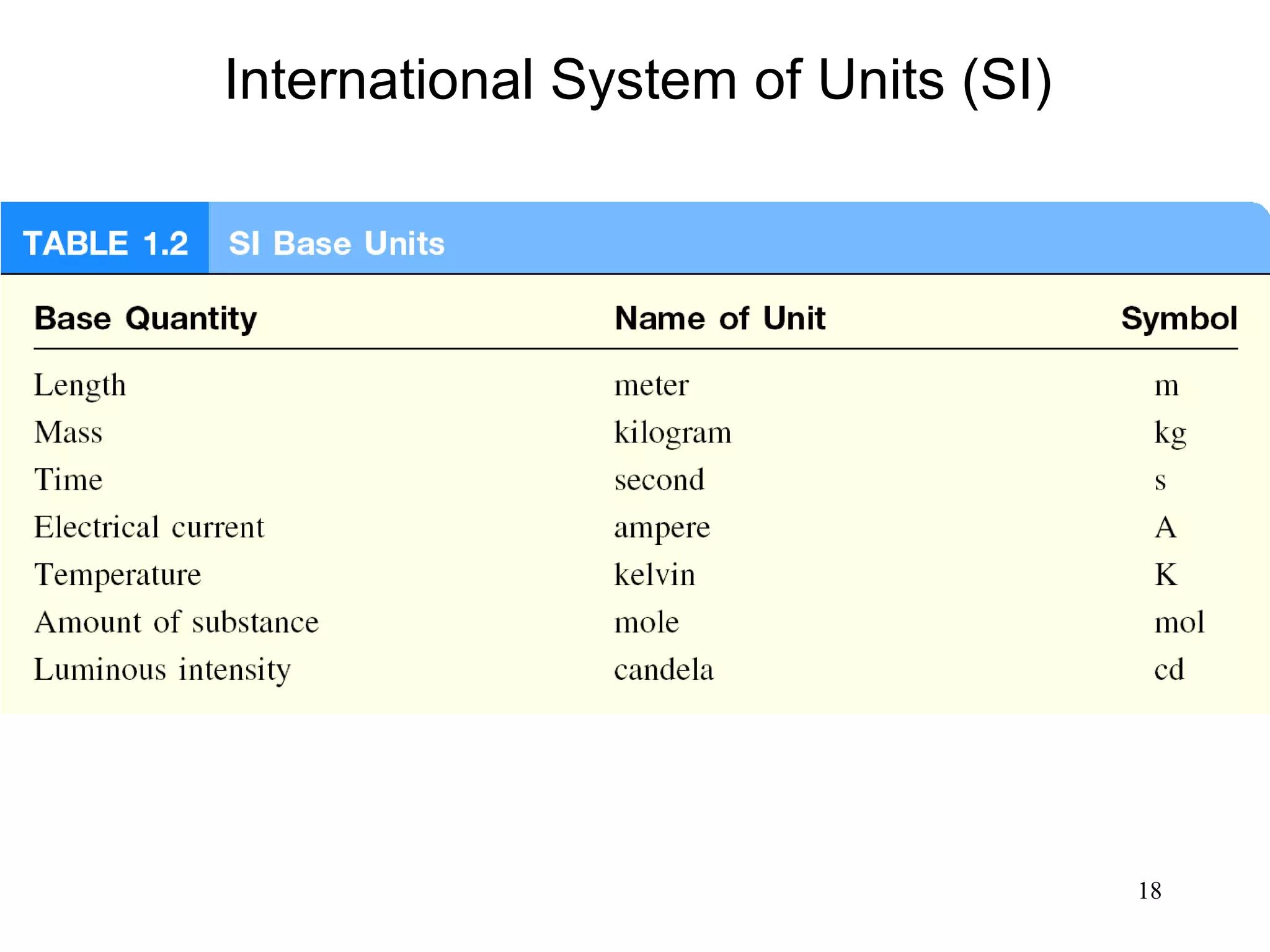
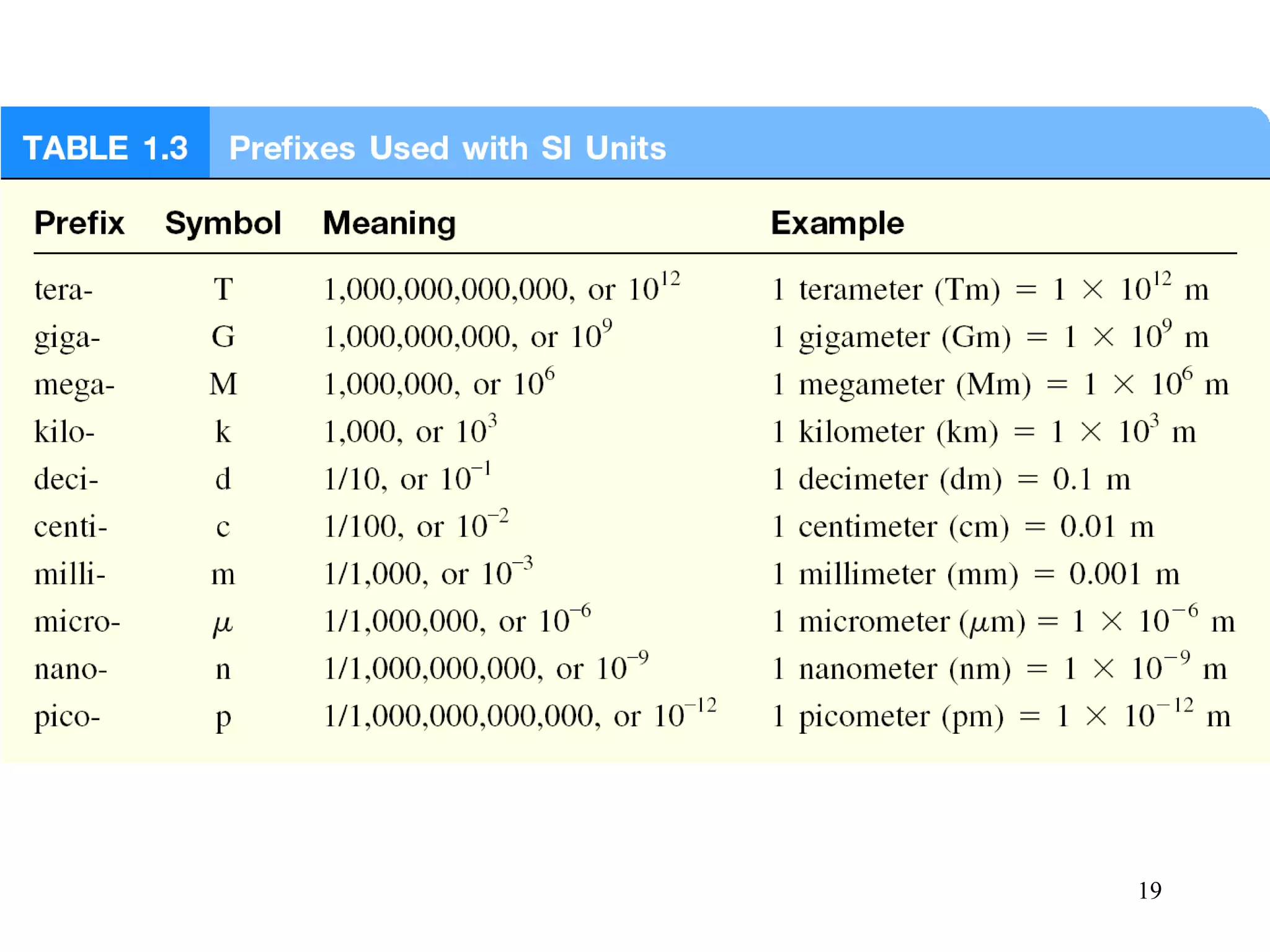
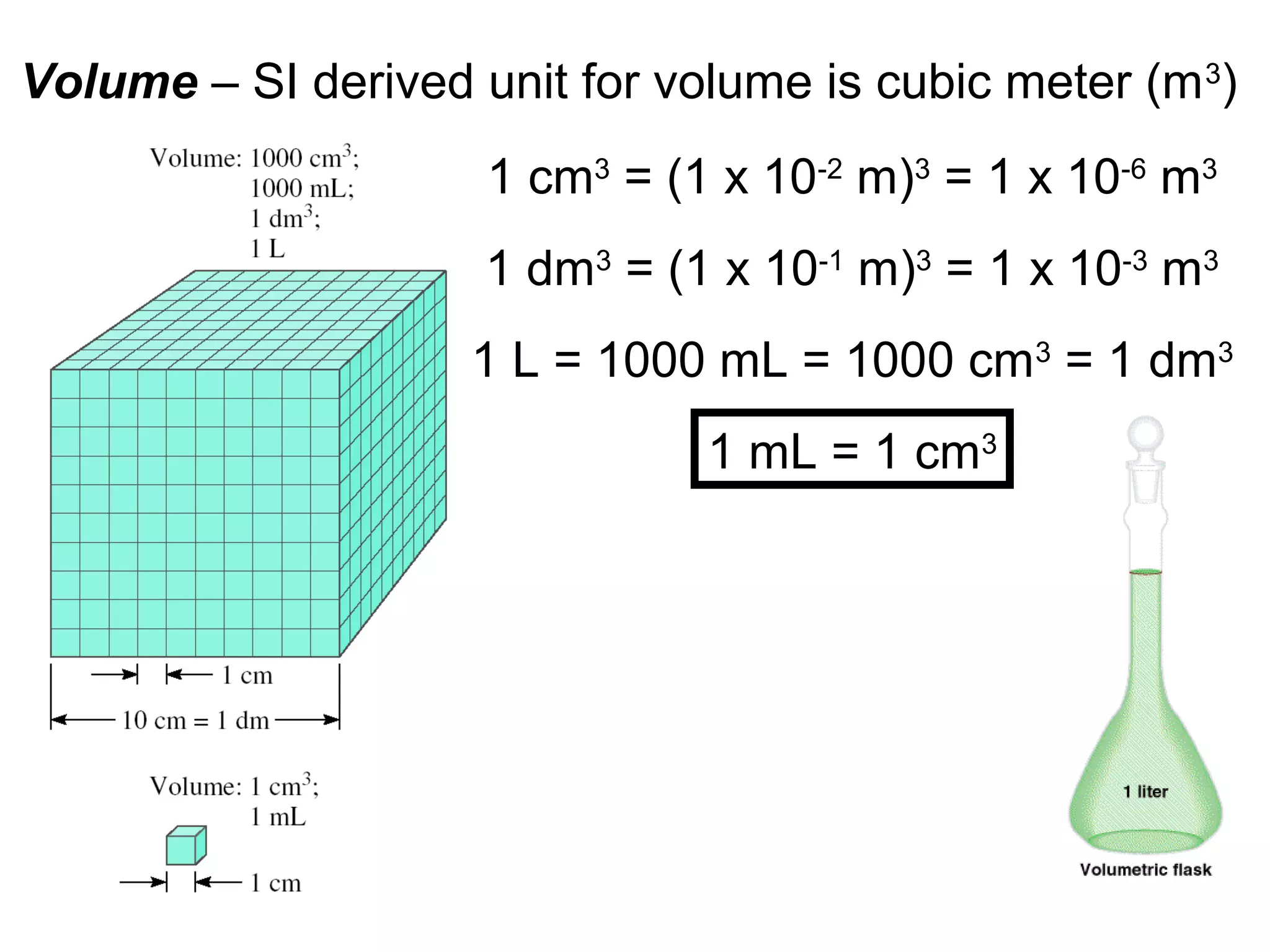
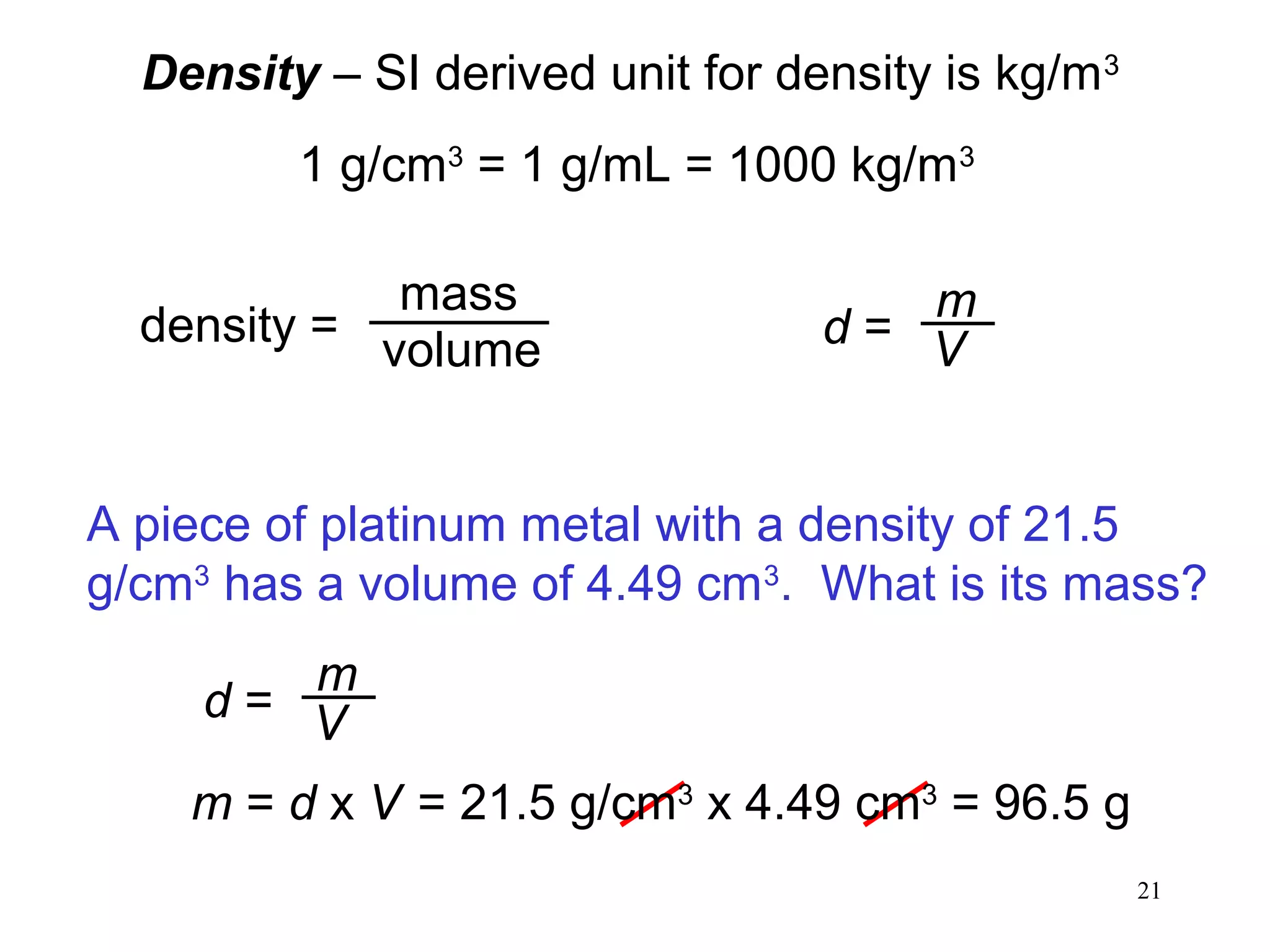
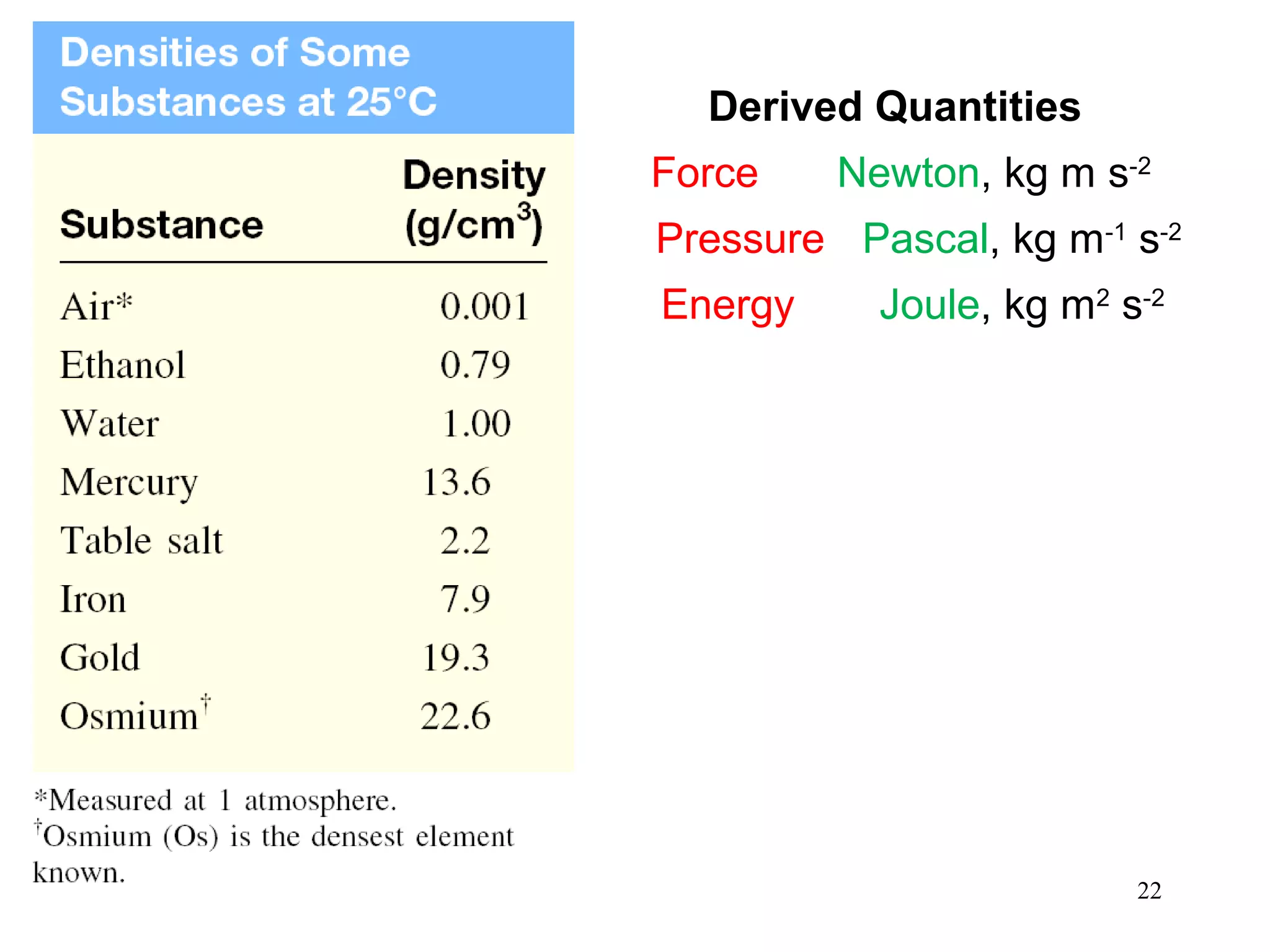
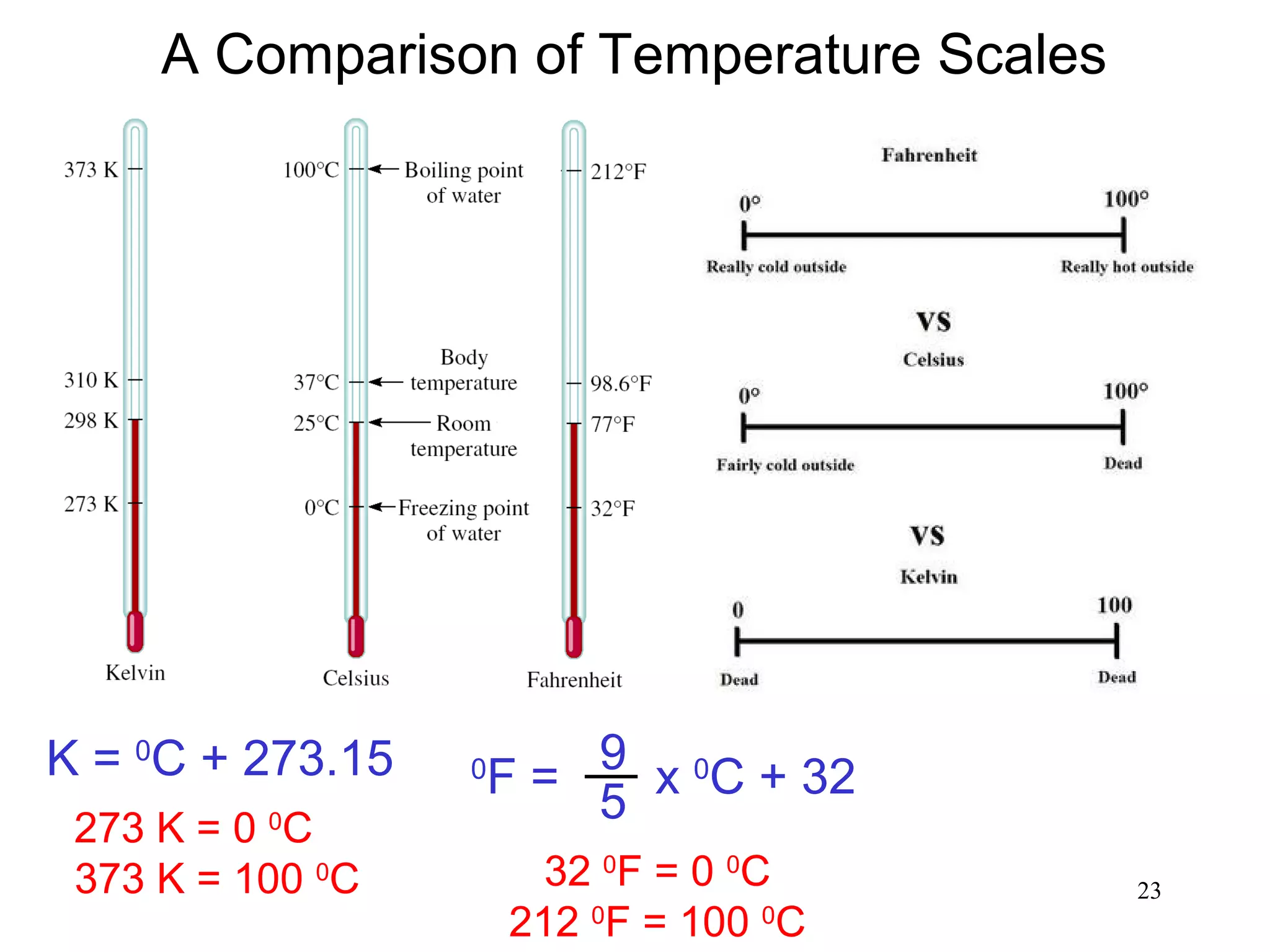
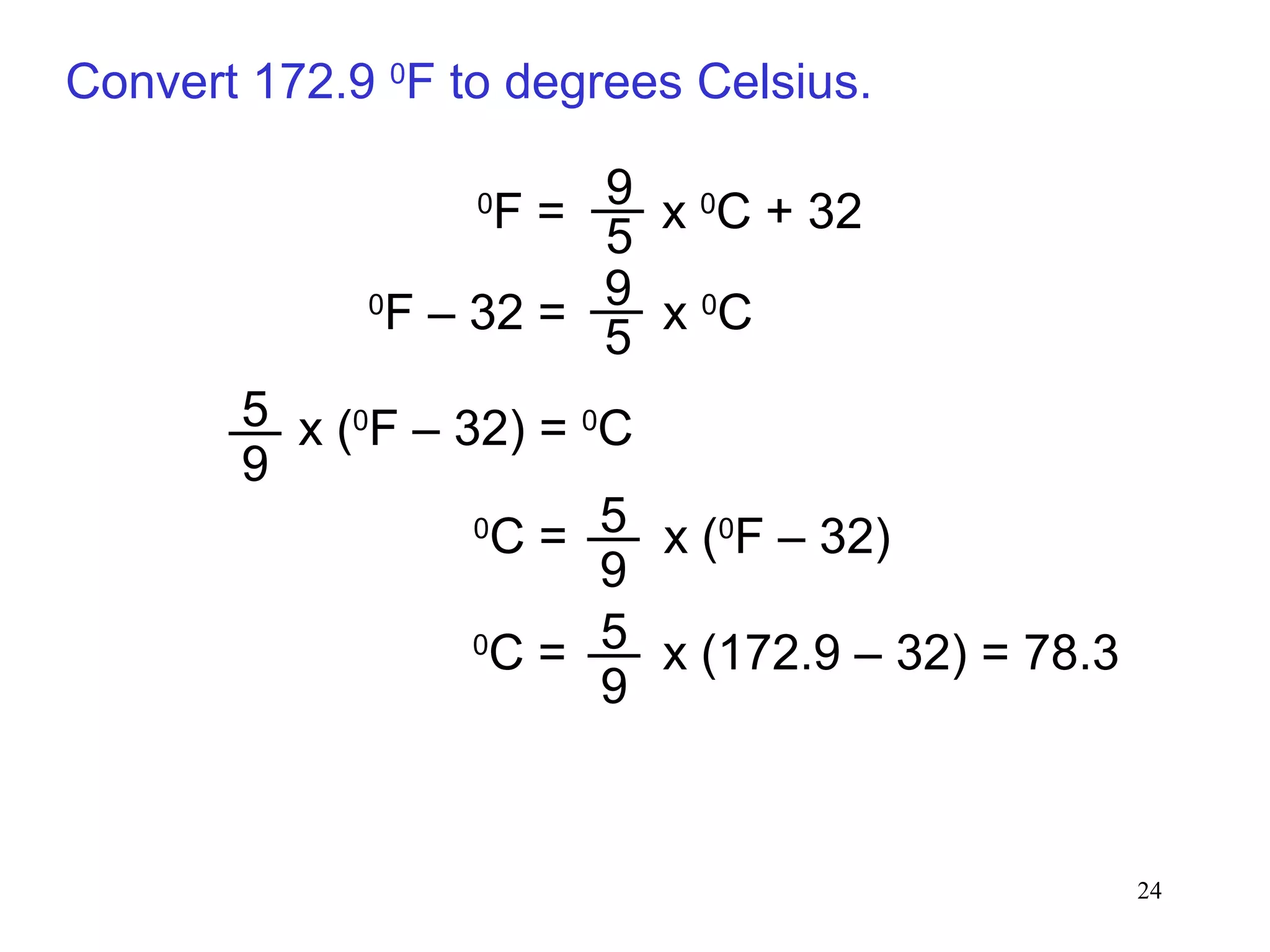
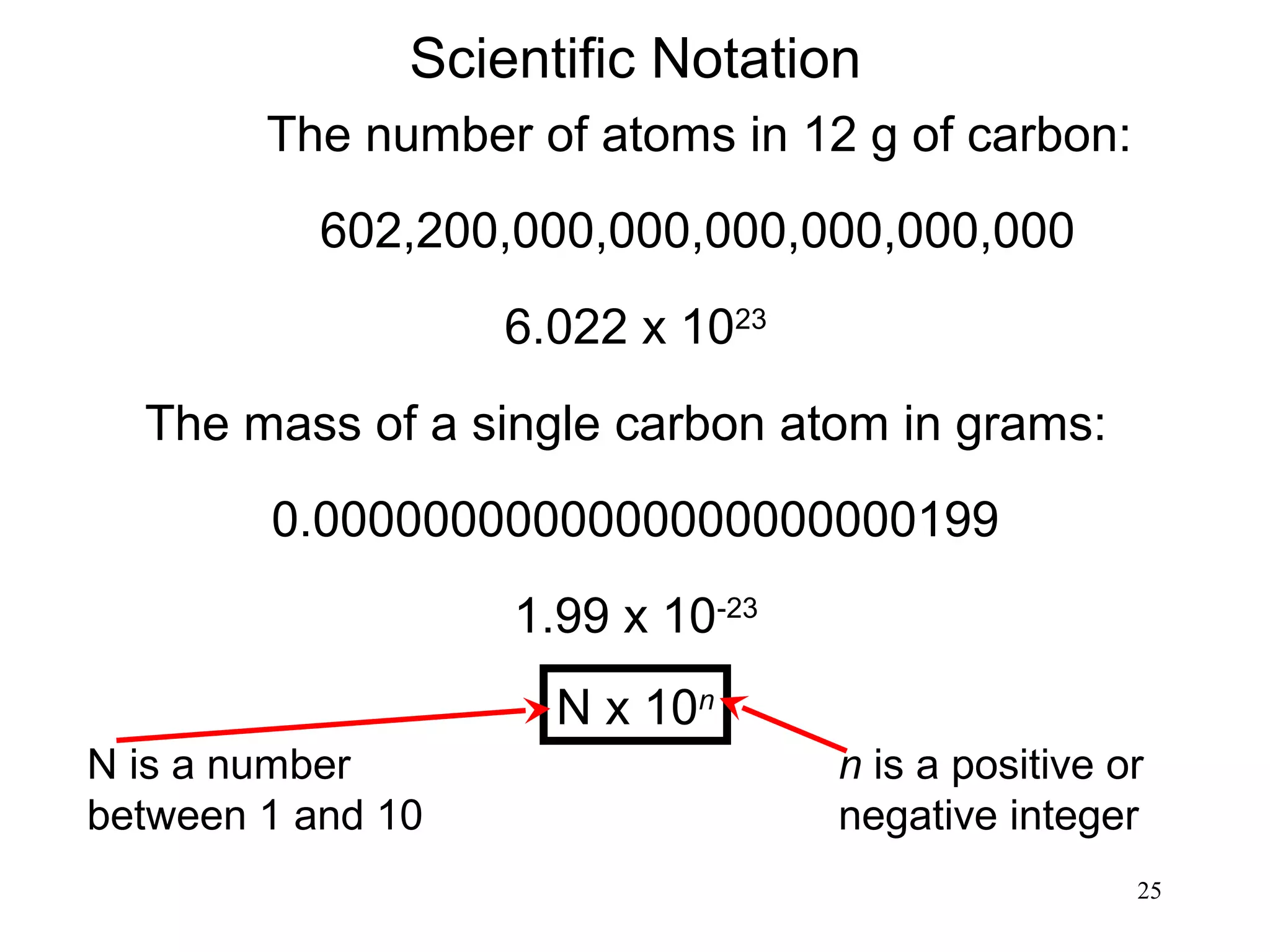
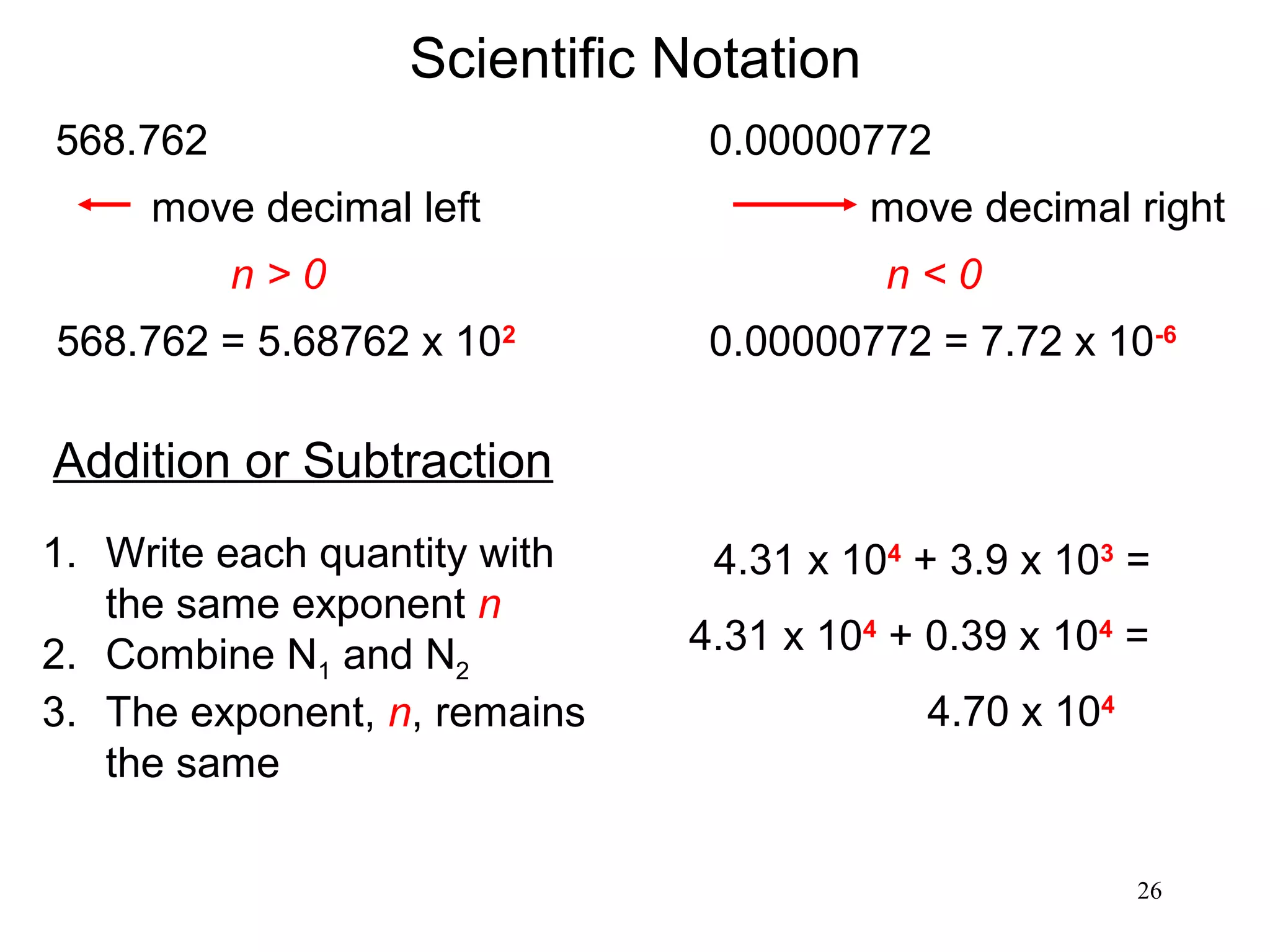
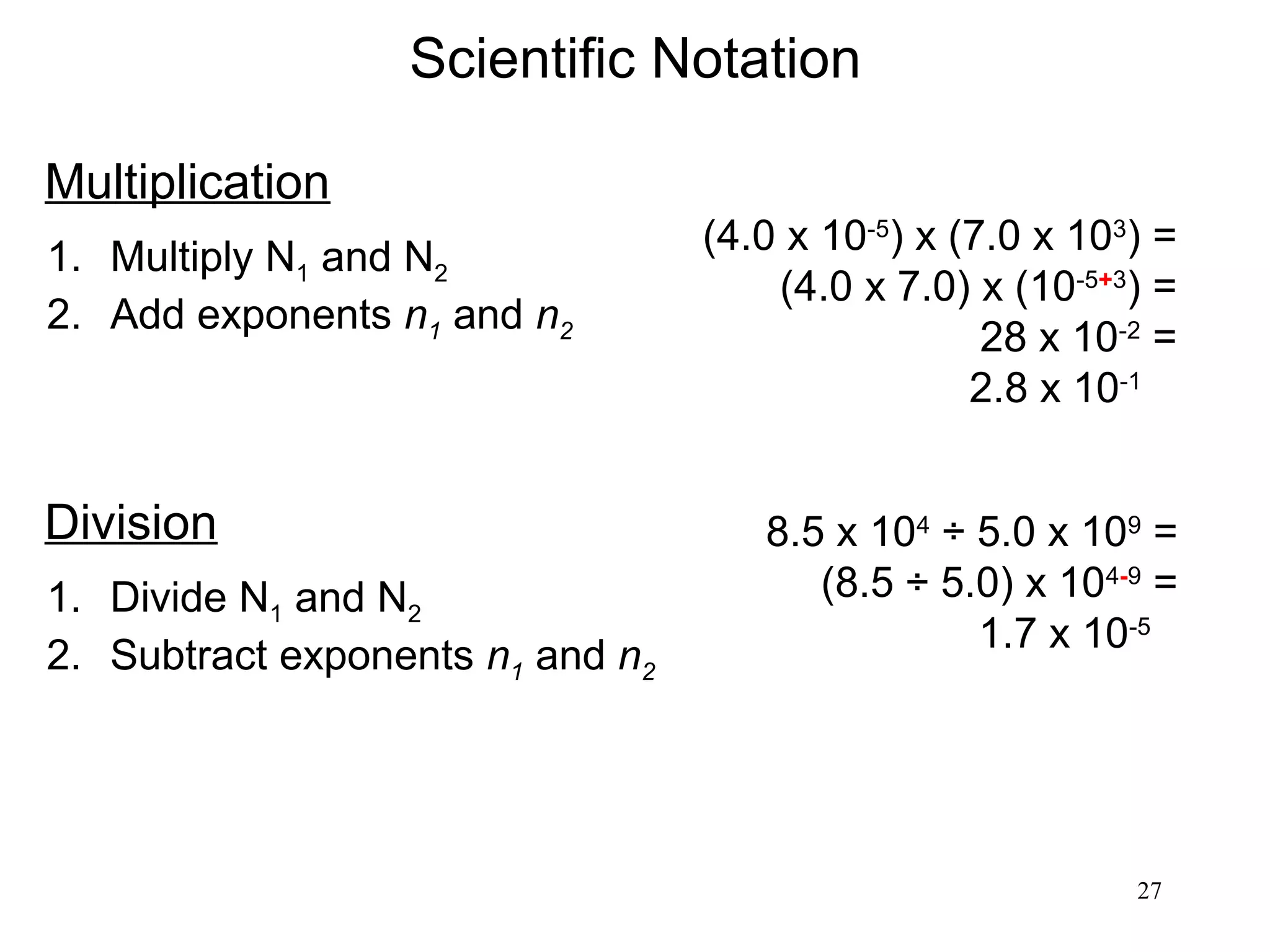
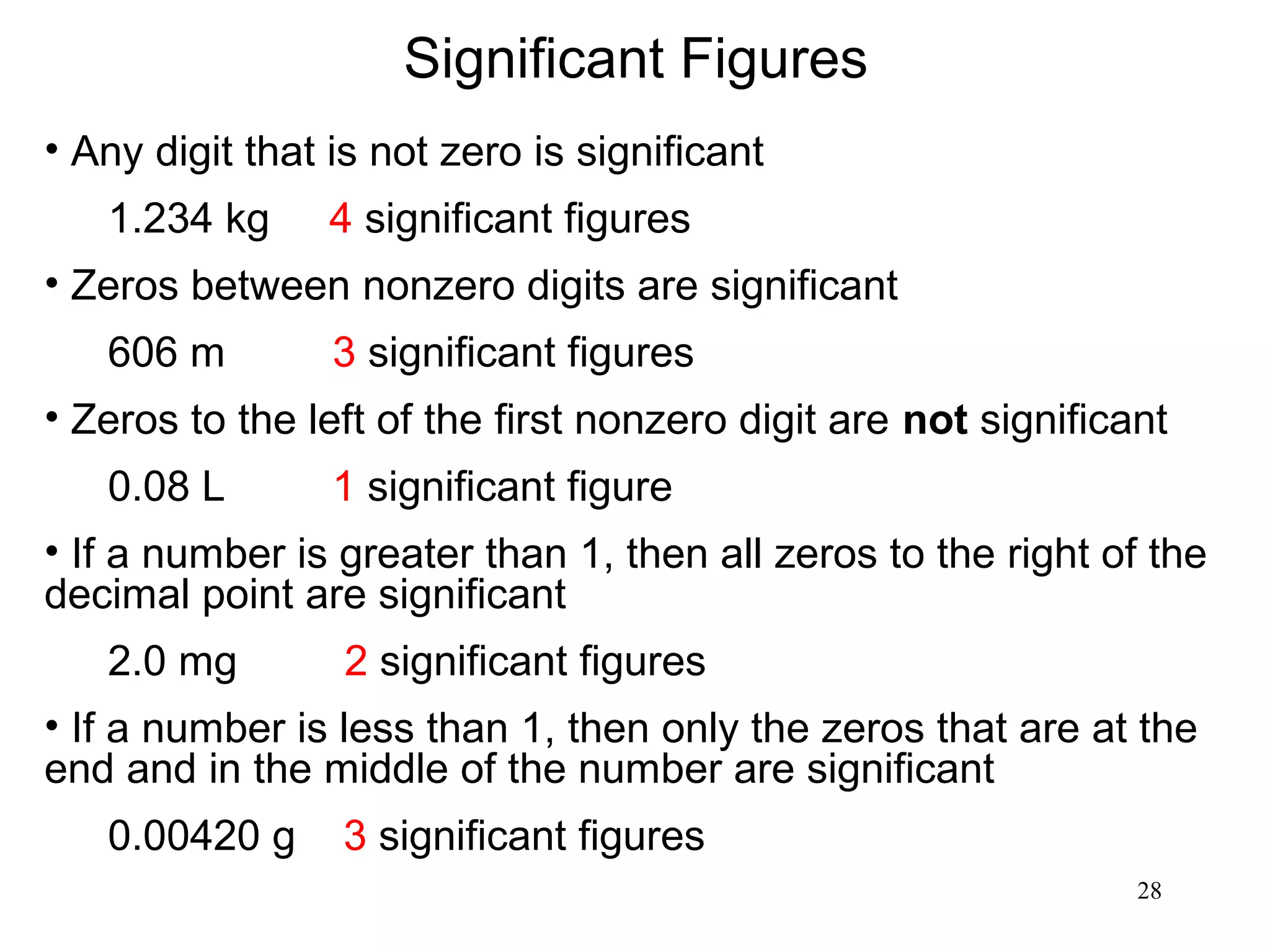
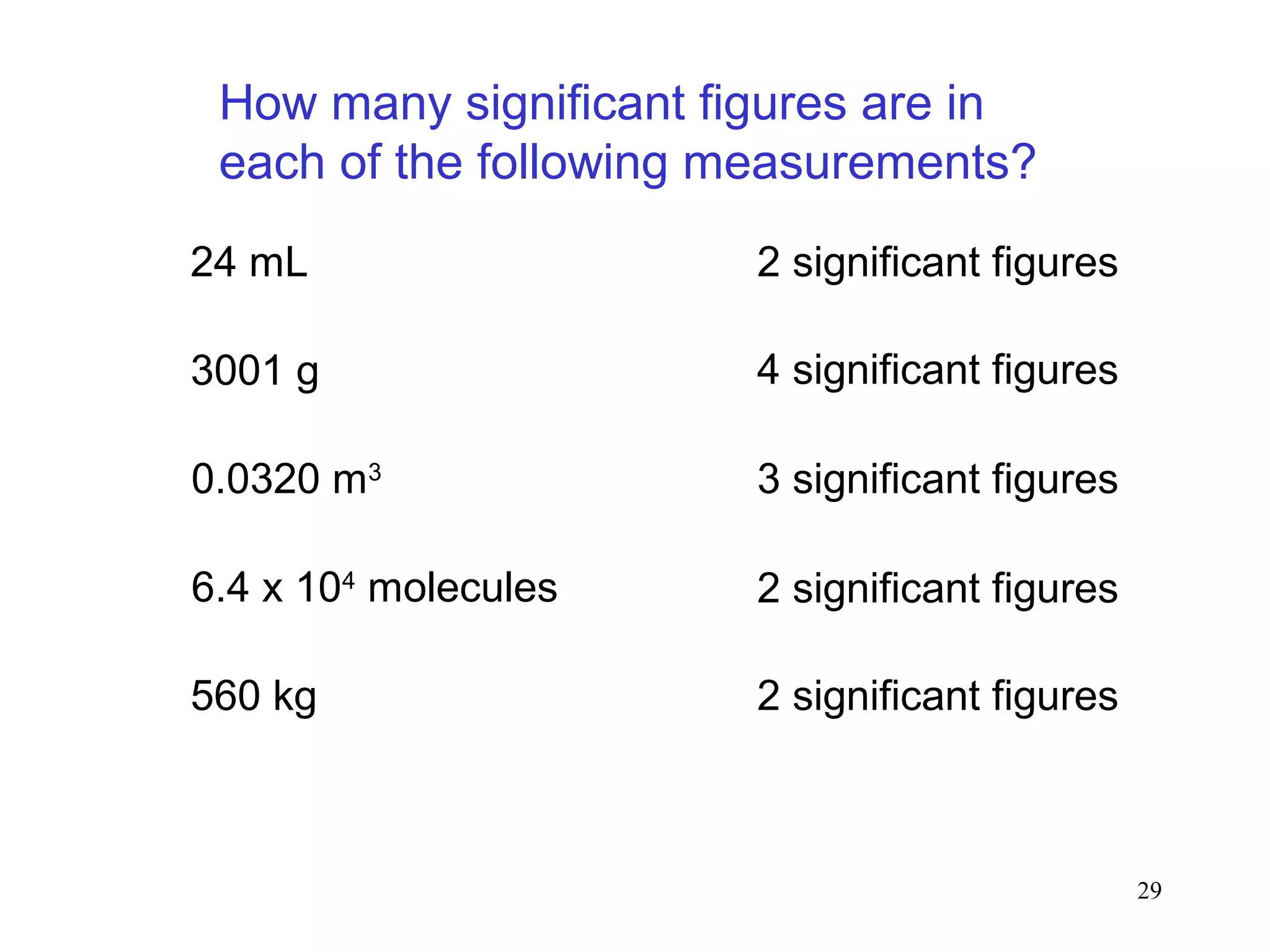
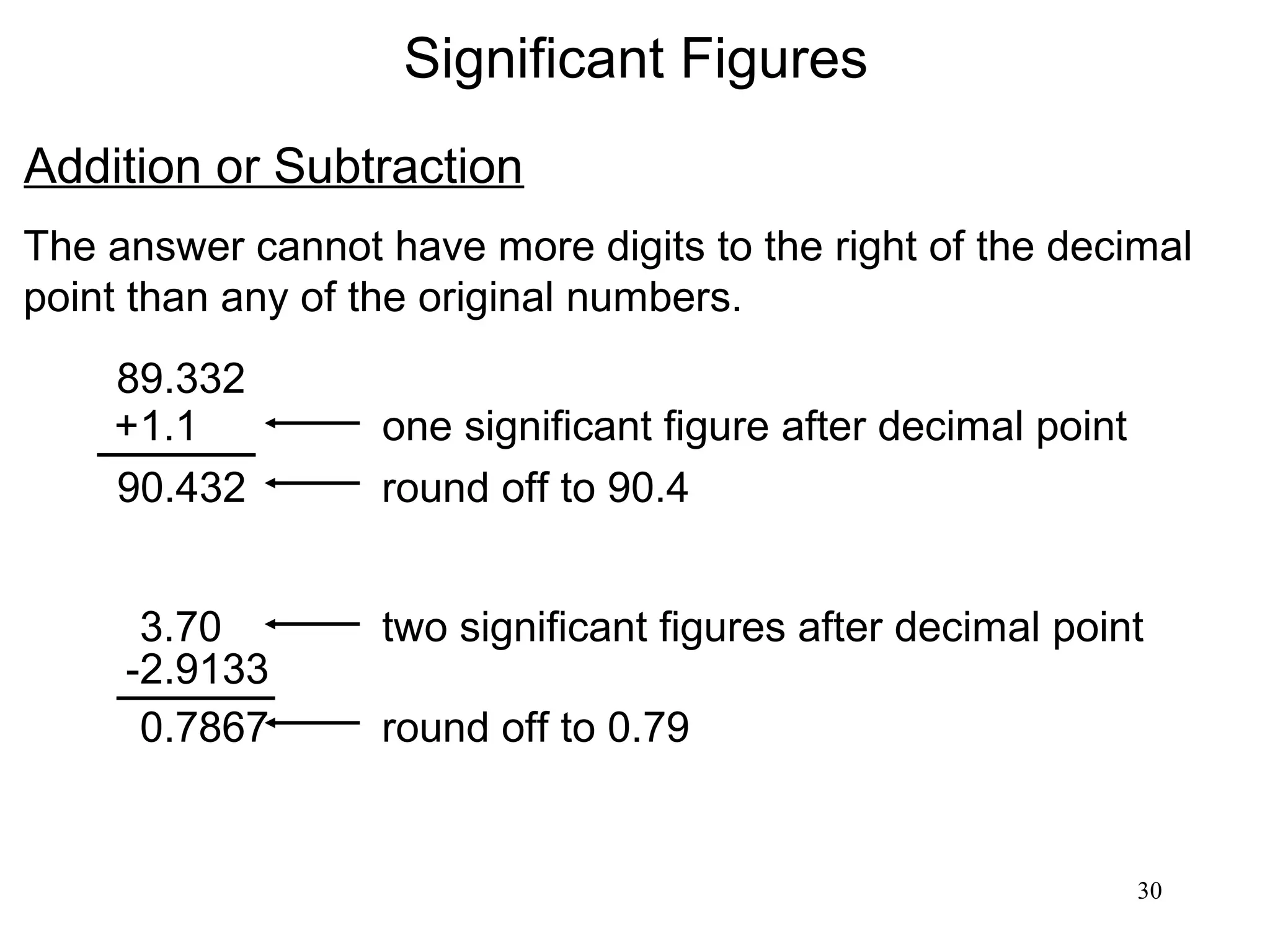
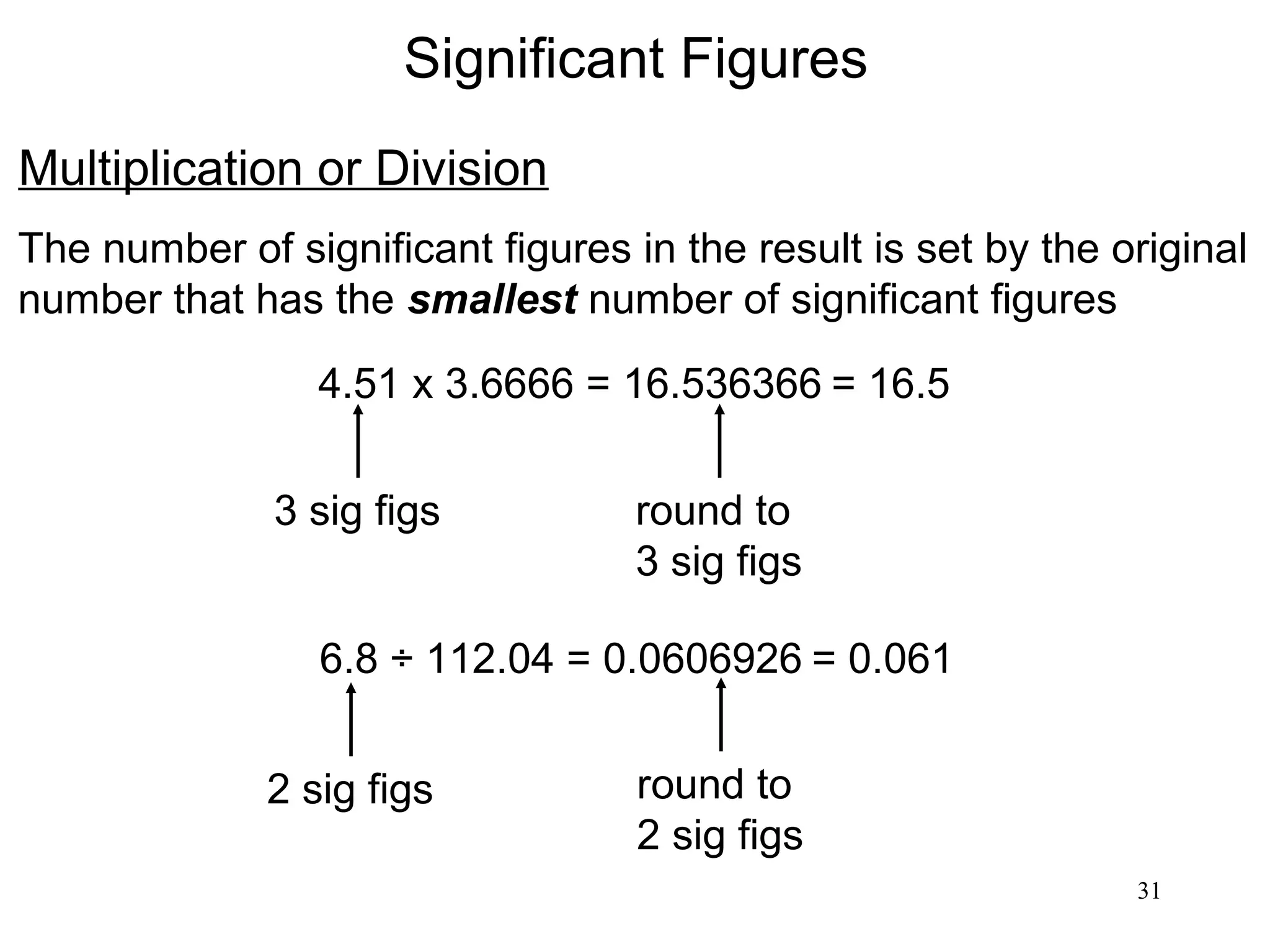
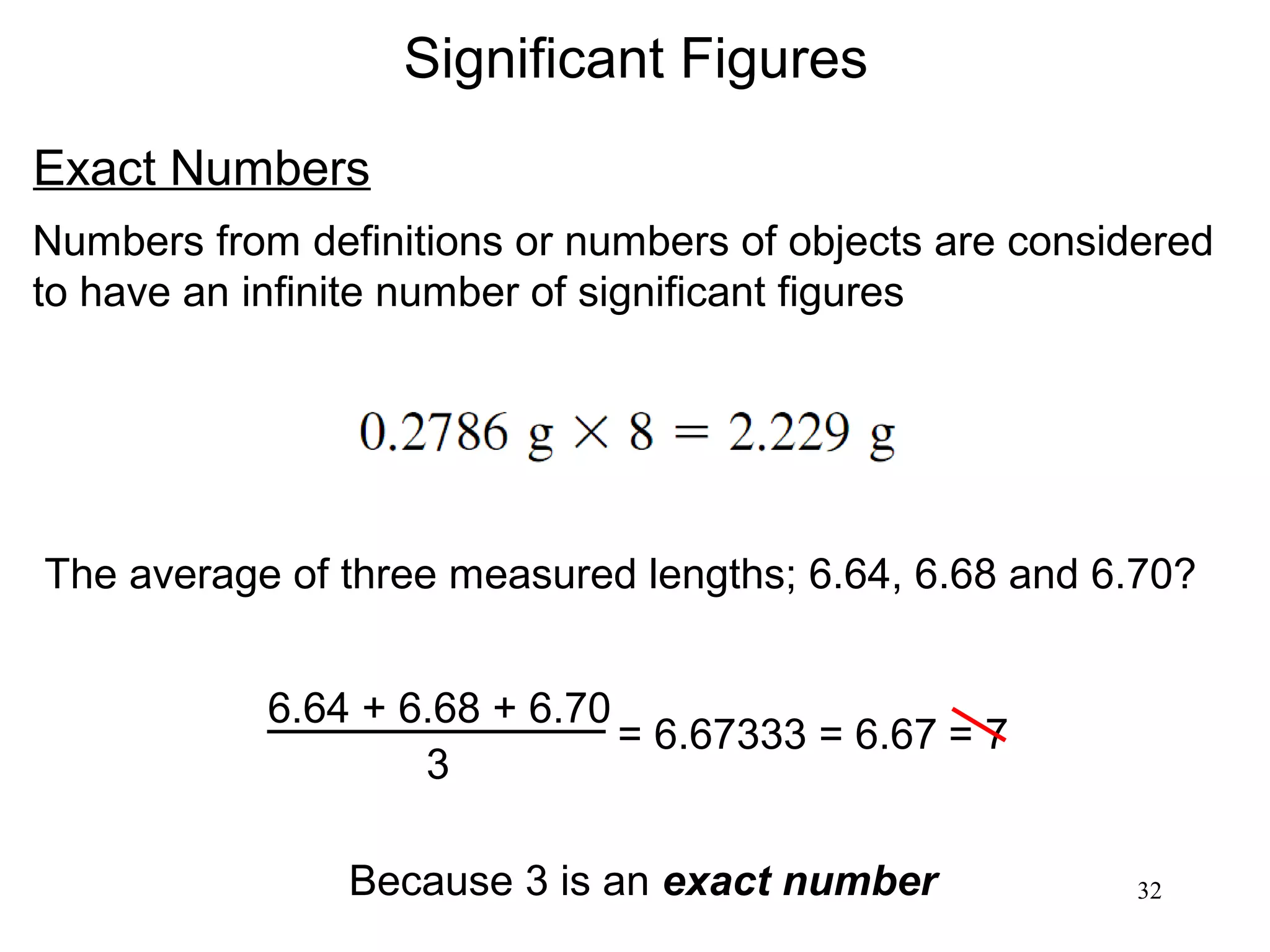
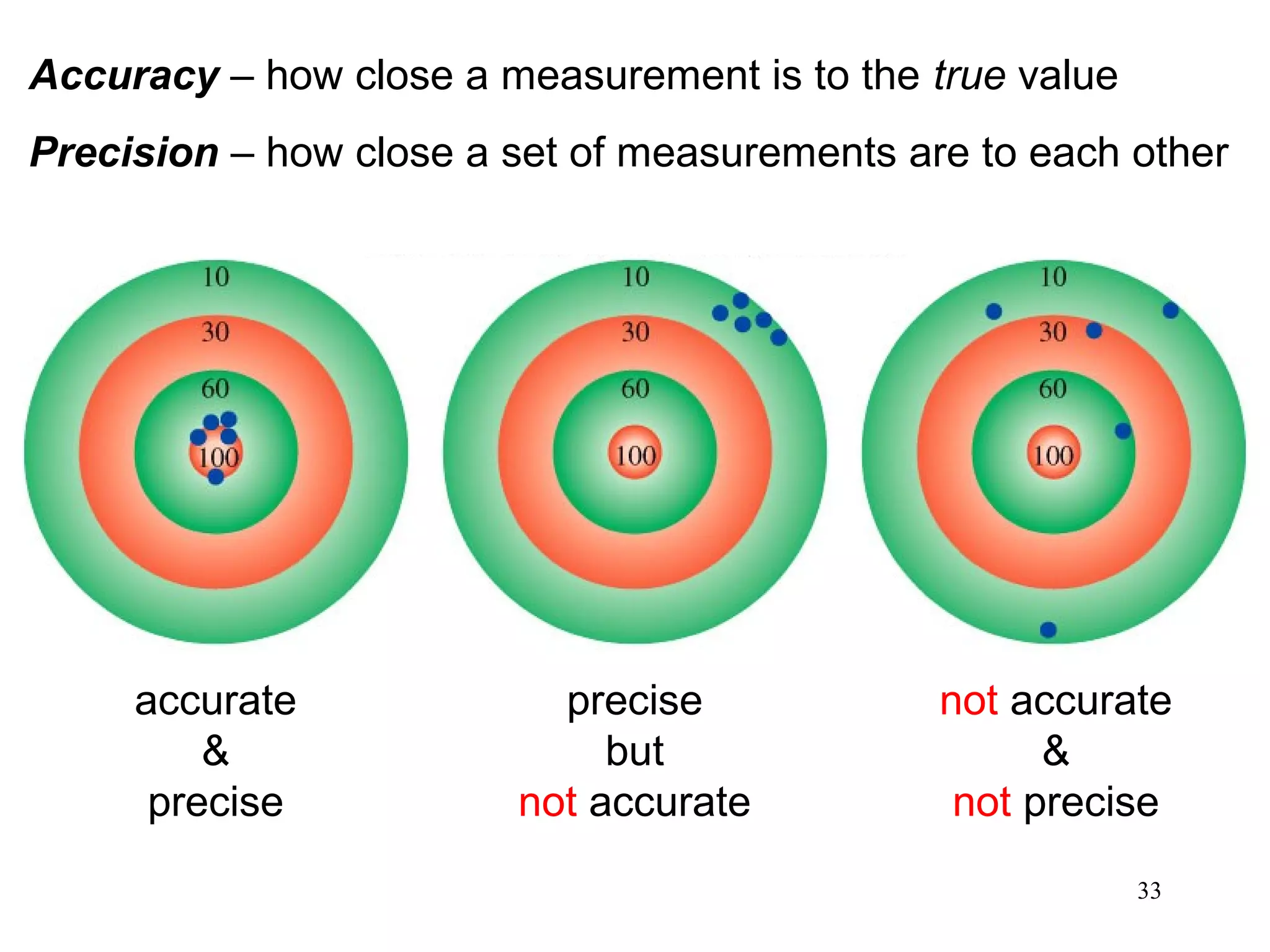
3. The study of chemistry incorporates macroscopic observations and measurements as well as analysis at the microscopic level of atoms and molecules. Significant figures, units, and mathematical representations are important tools in chemistry.