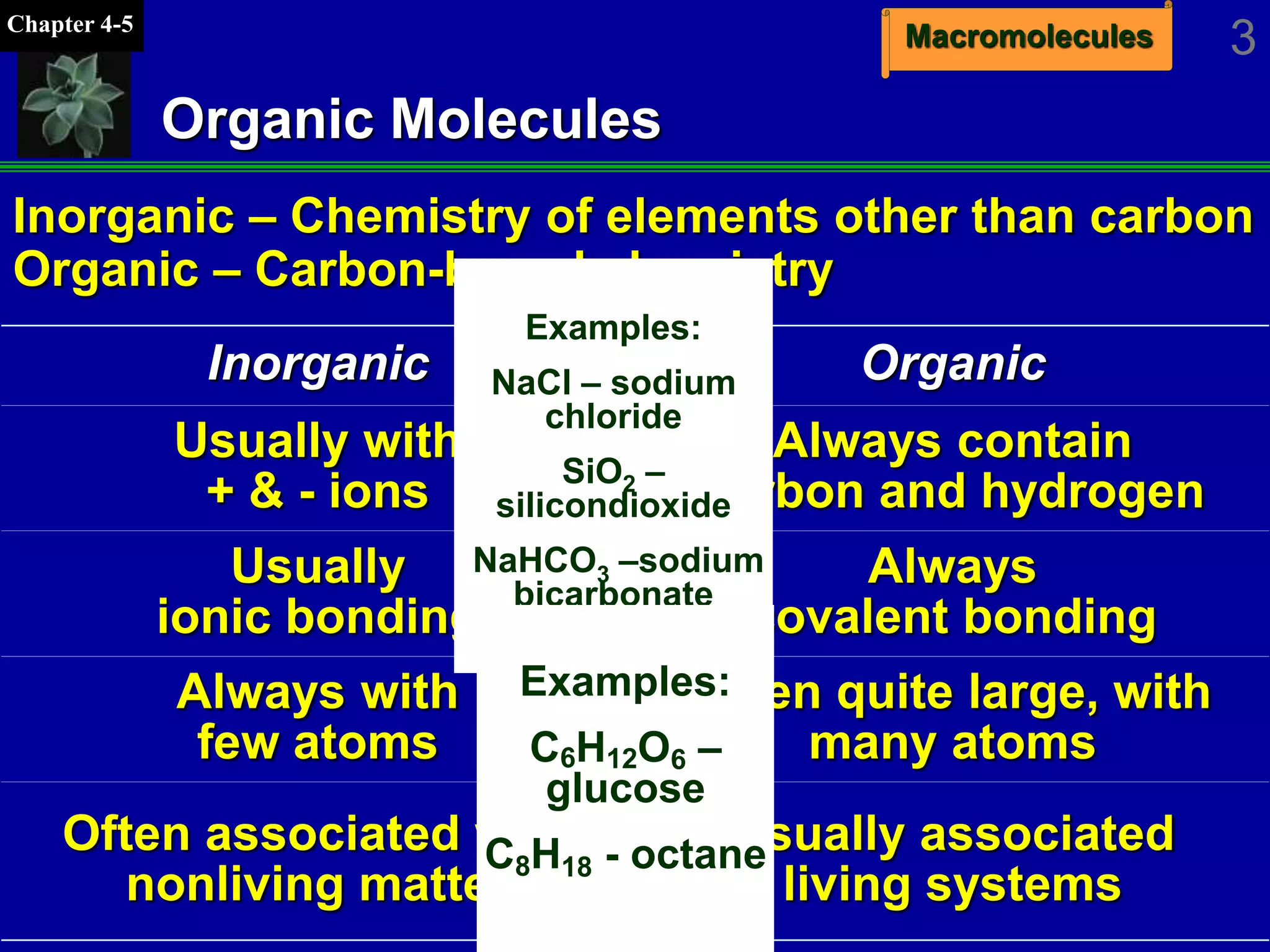
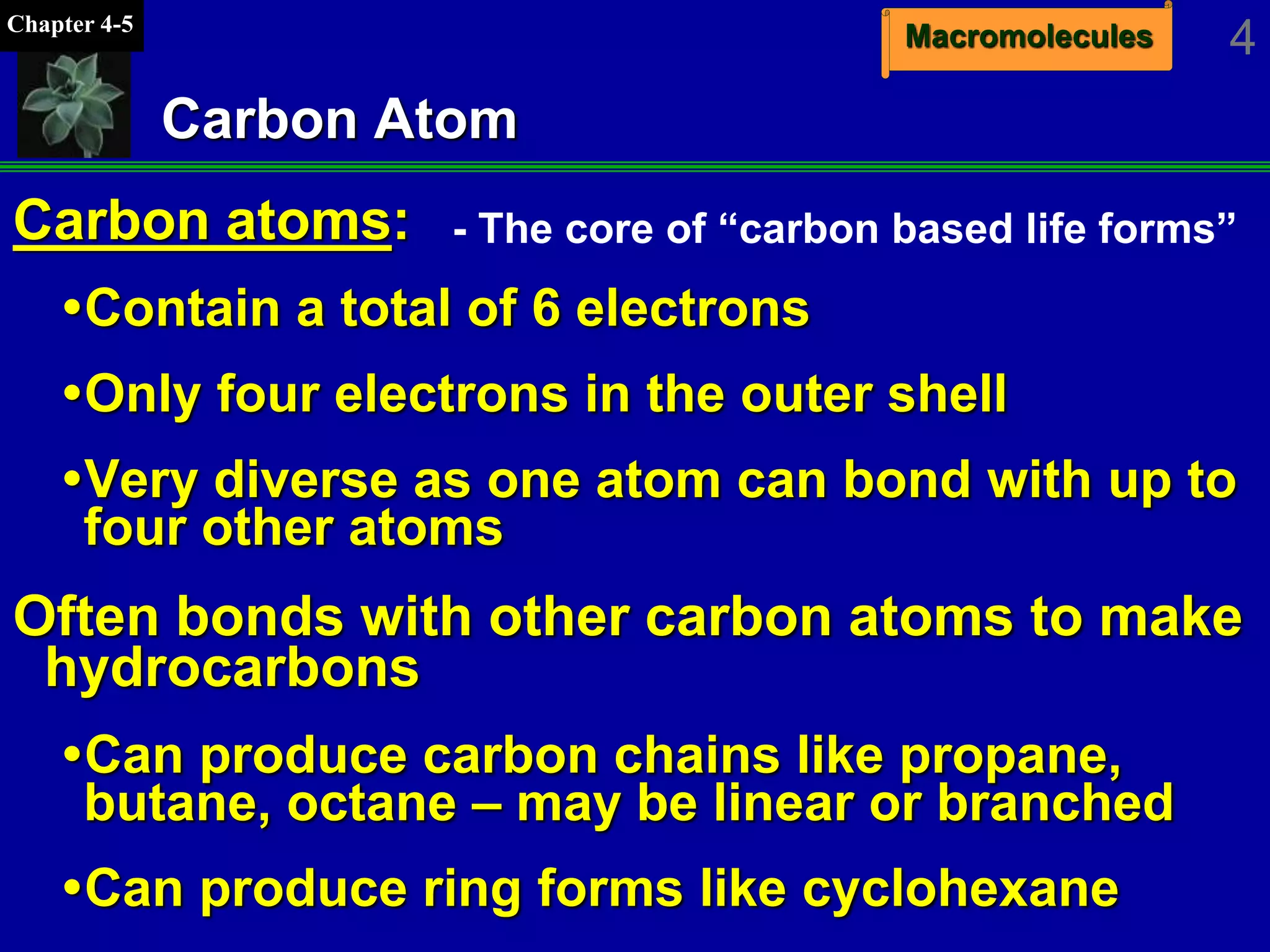
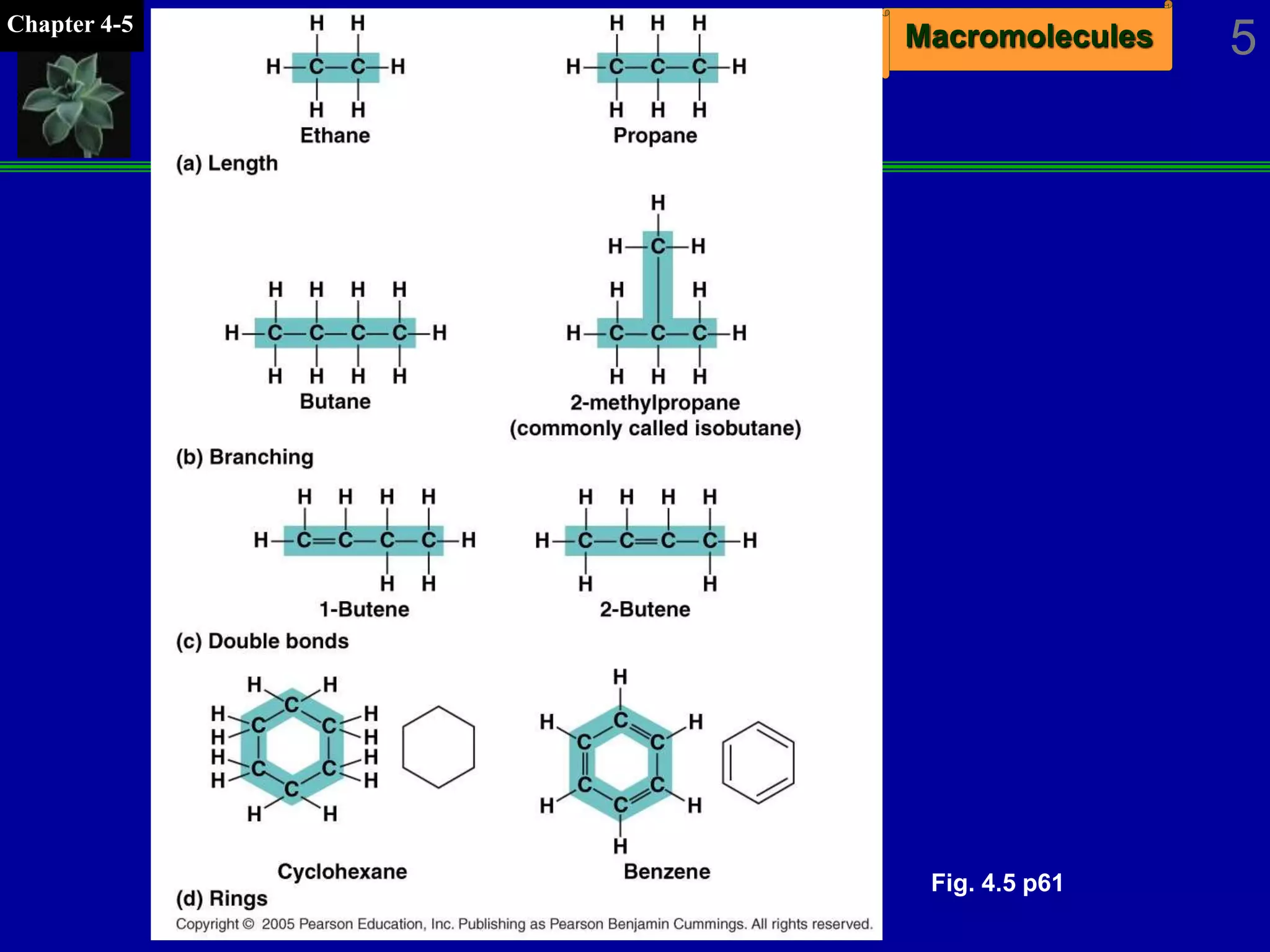
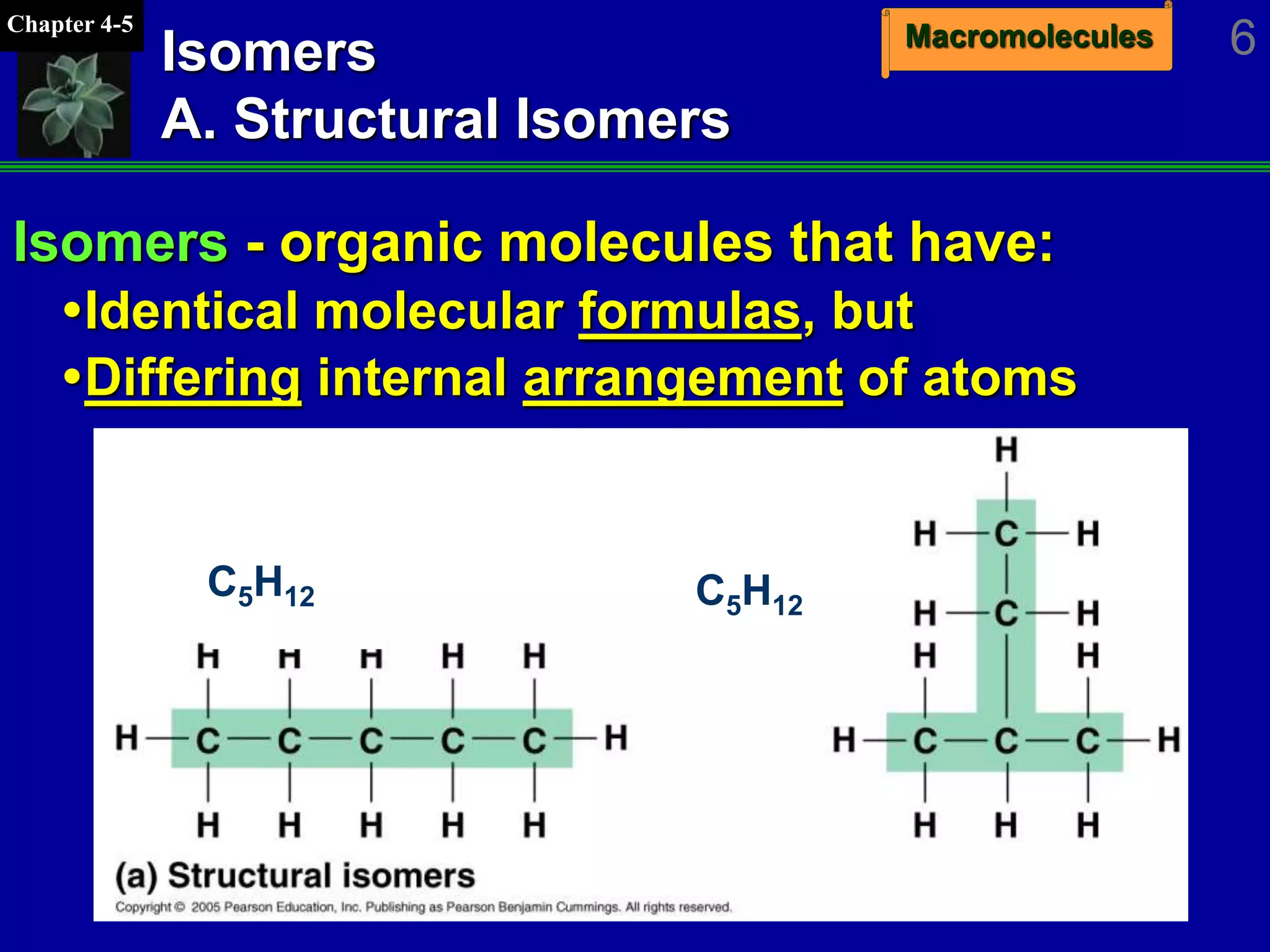
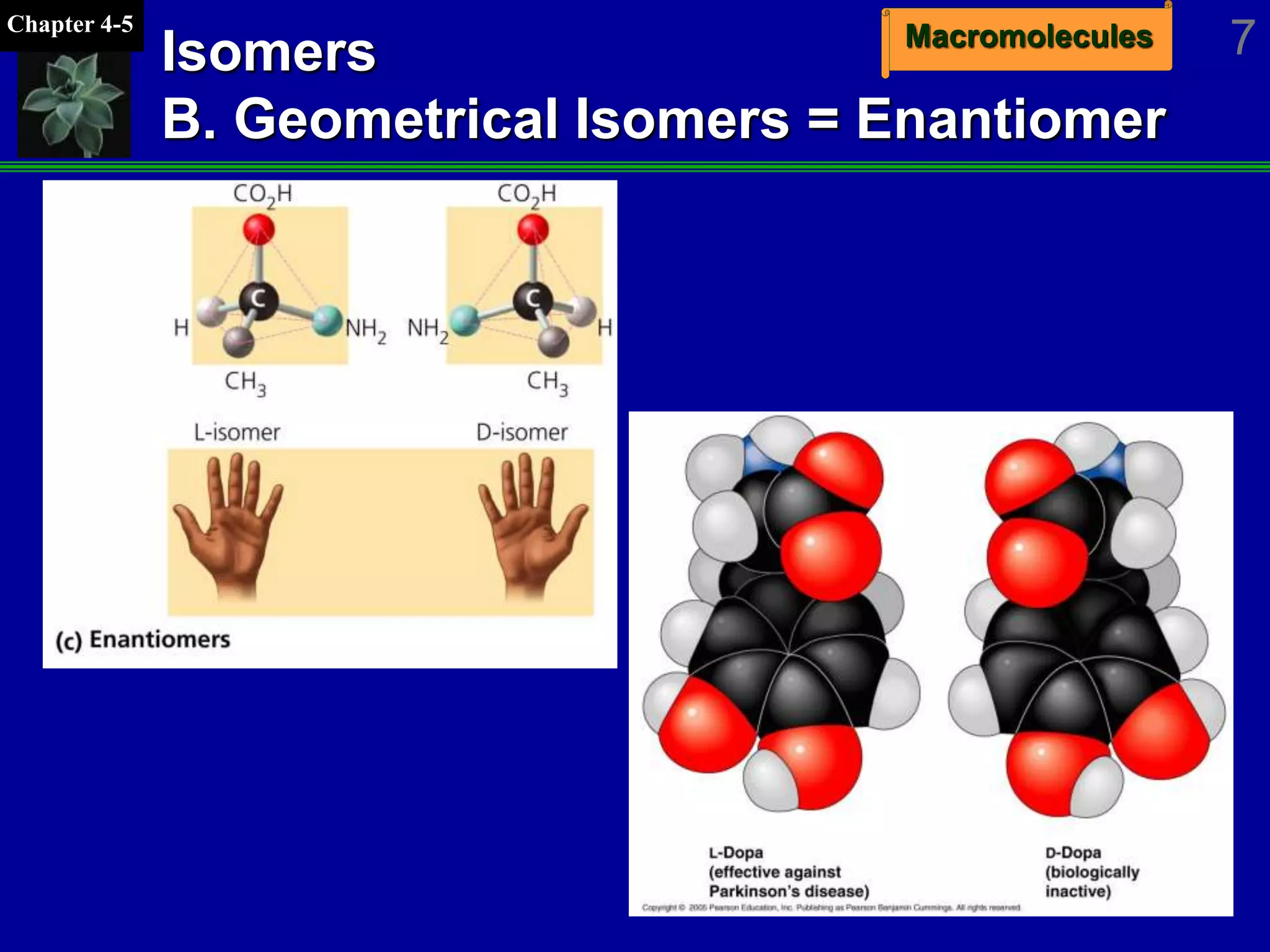
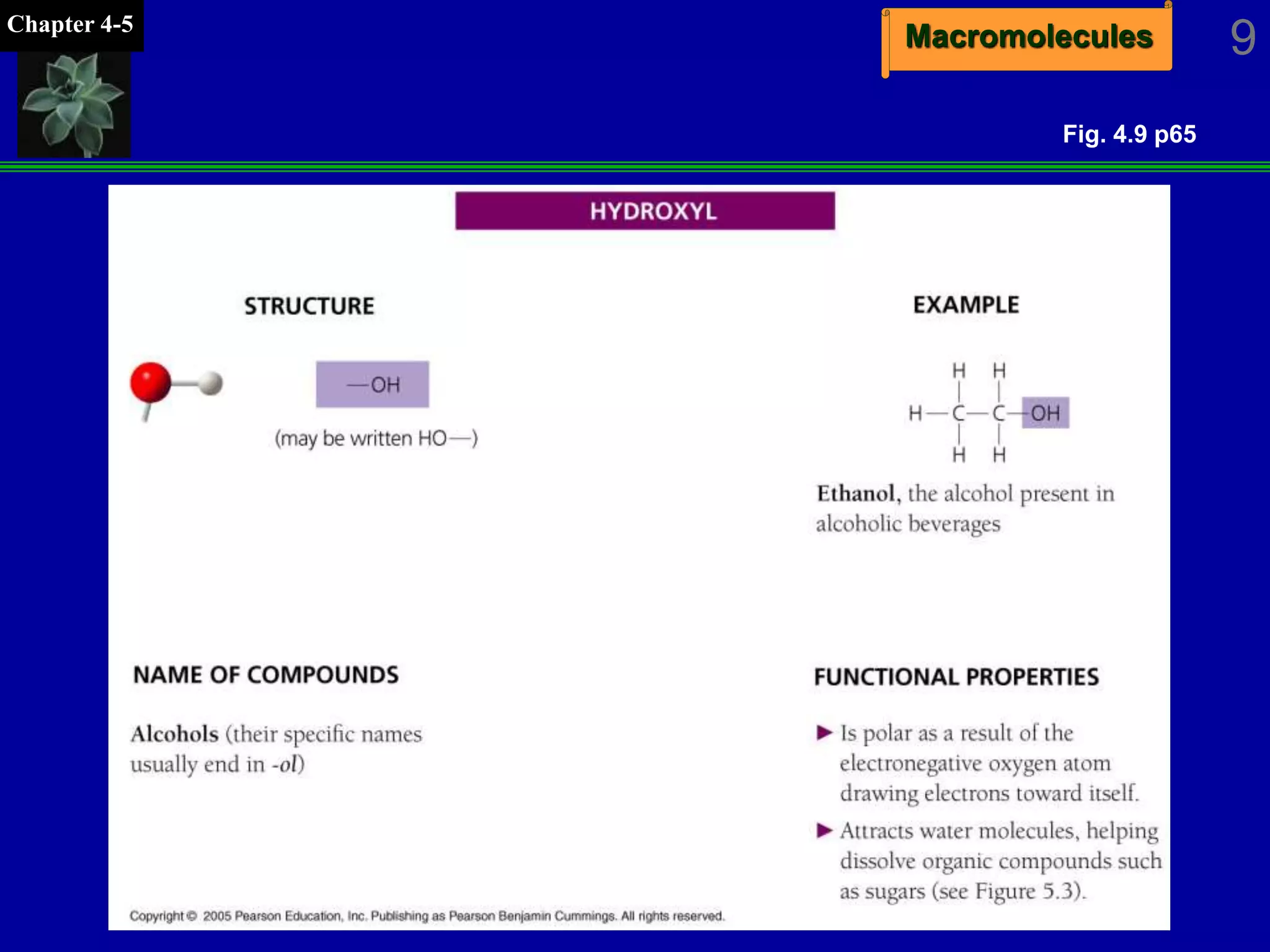
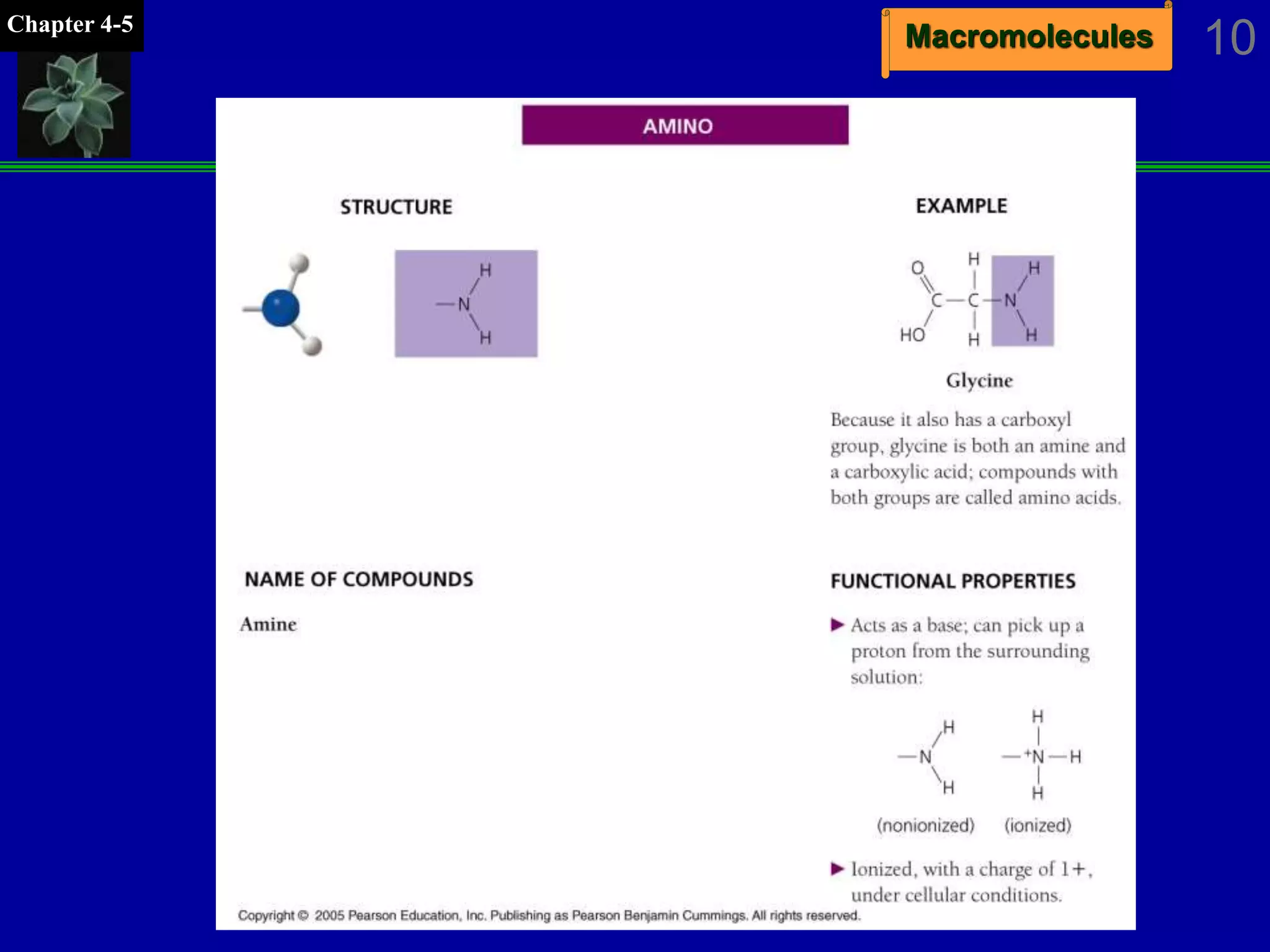
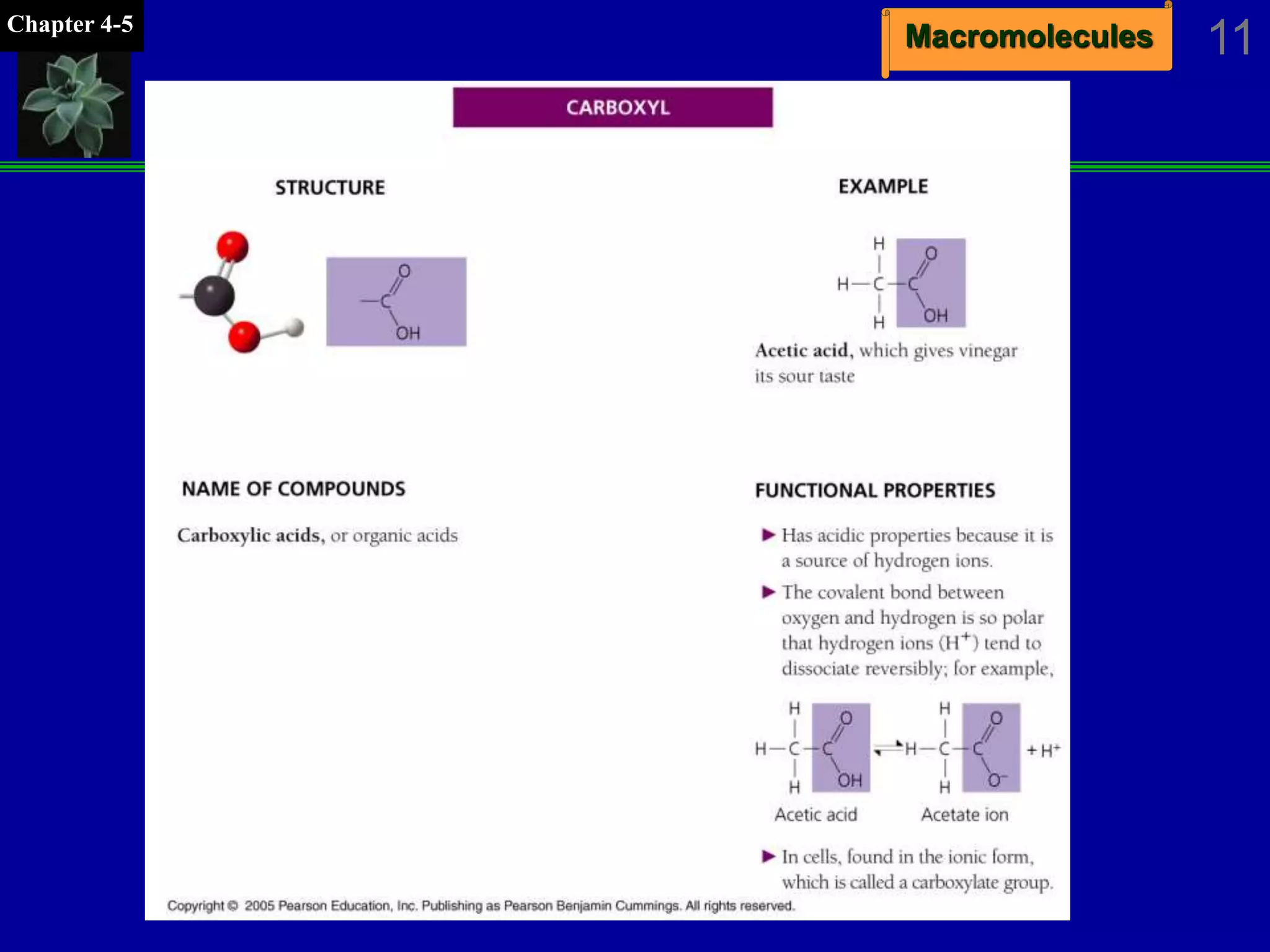
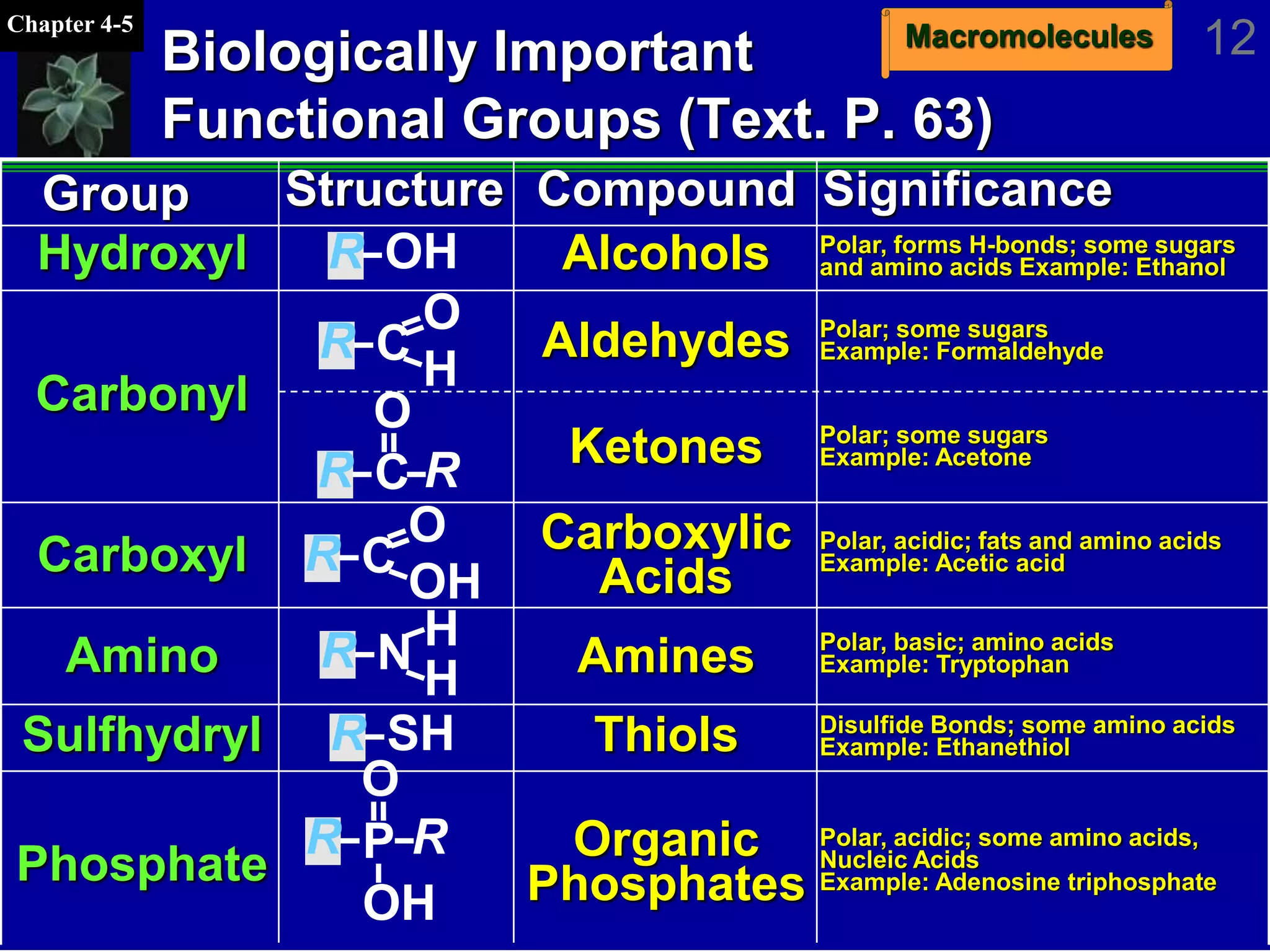
This document discusses macromolecules and their structure and function. It outlines the four major classes of macromolecules - carbohydrates, lipids, proteins, and nucleic acids. It explains that organic molecules contain carbon and are associated with living systems, while inorganic molecules do not contain carbon and are associated with nonliving matter. The document discusses carbon atoms and their ability to form chains and rings, as well as isomers and important functional groups that determine molecular polarity and reactivity.