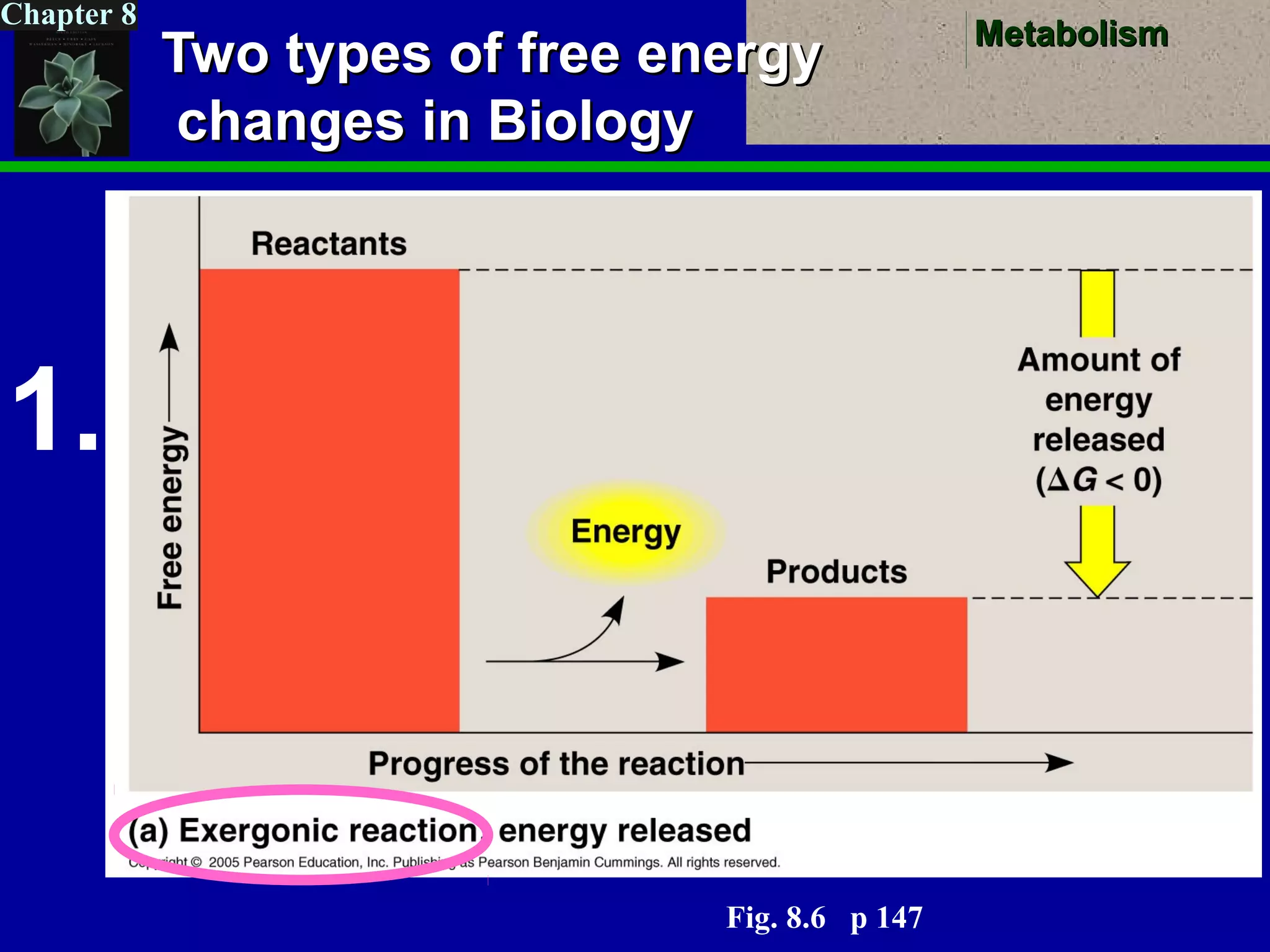
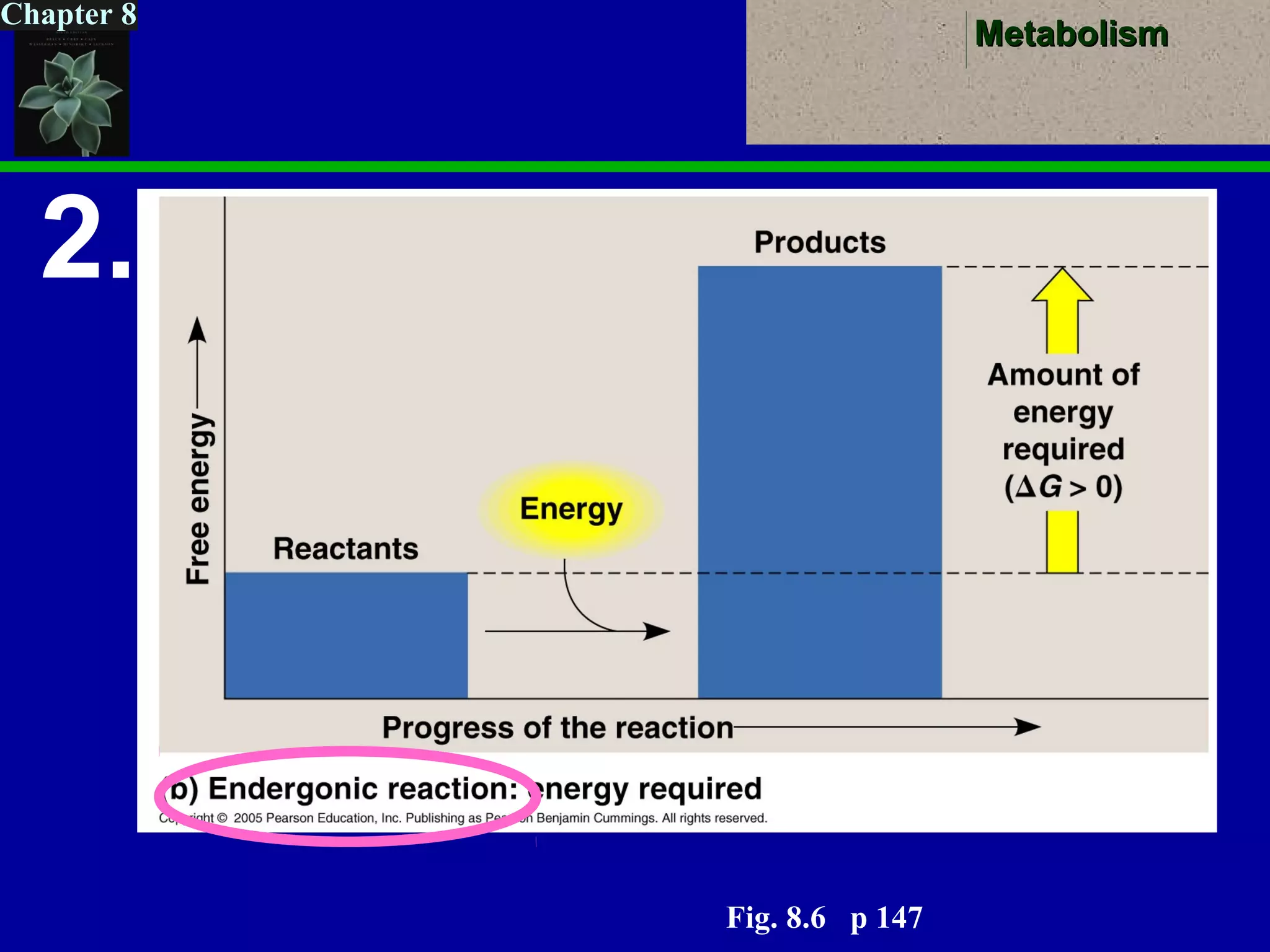
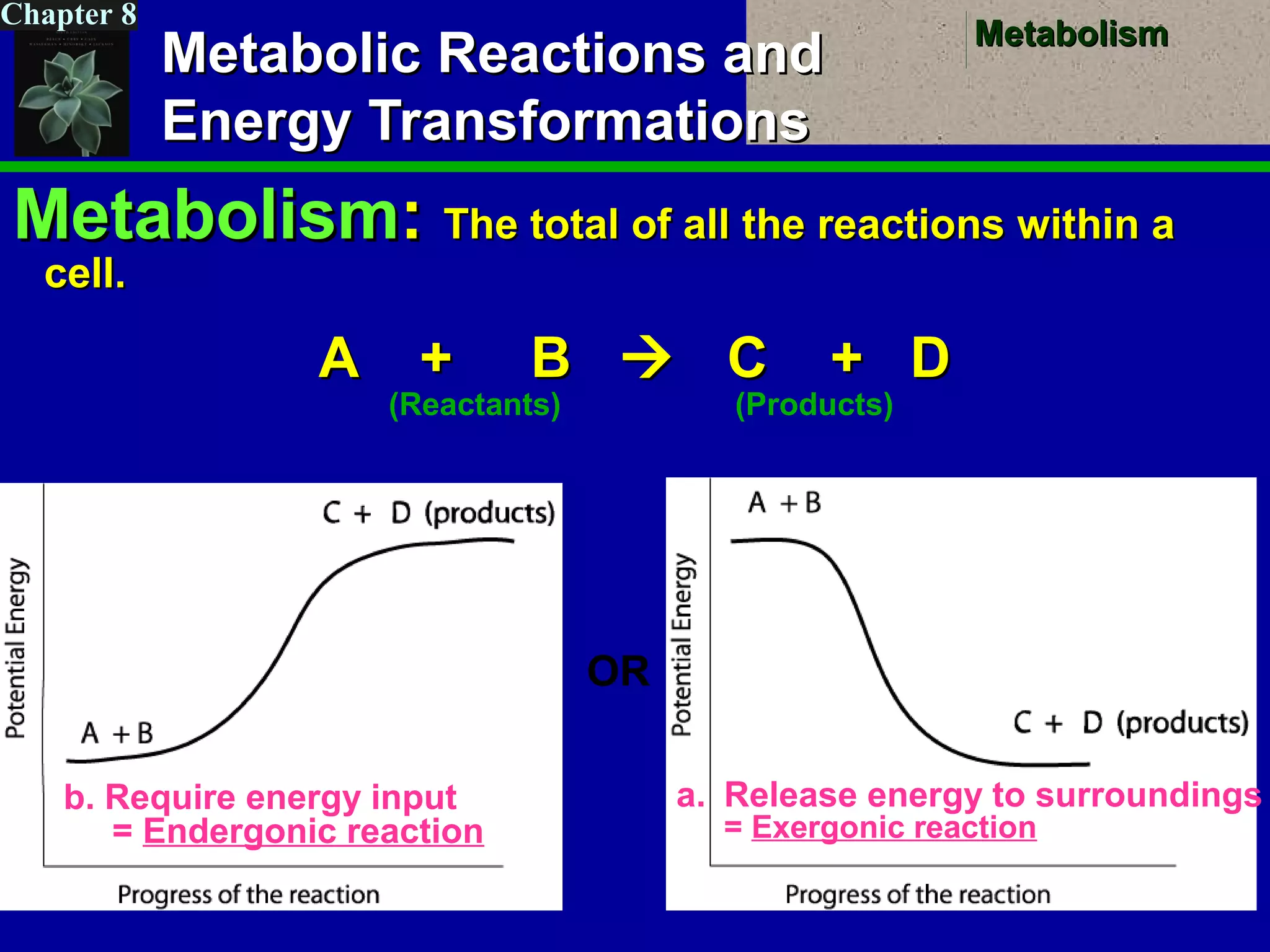
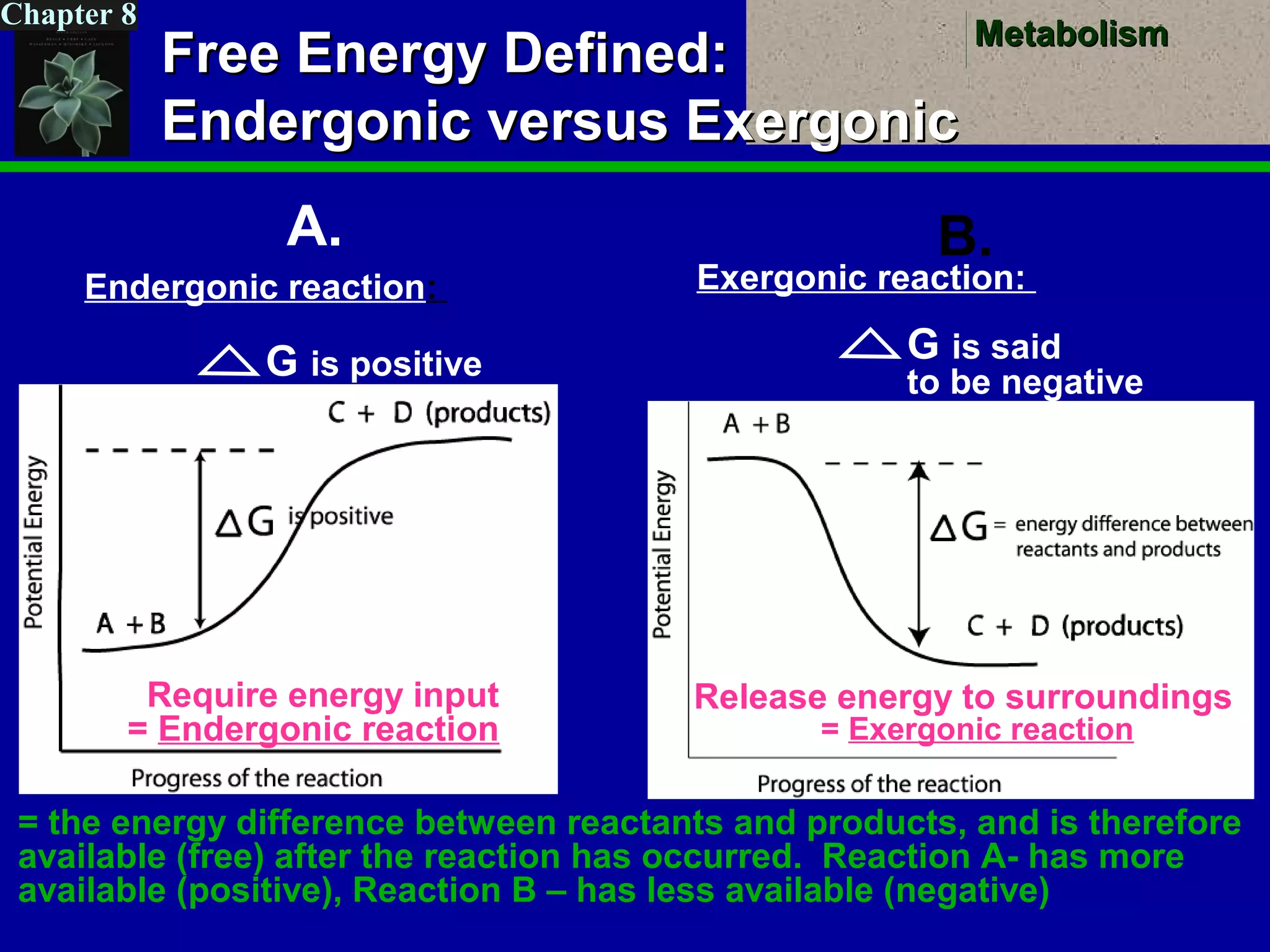
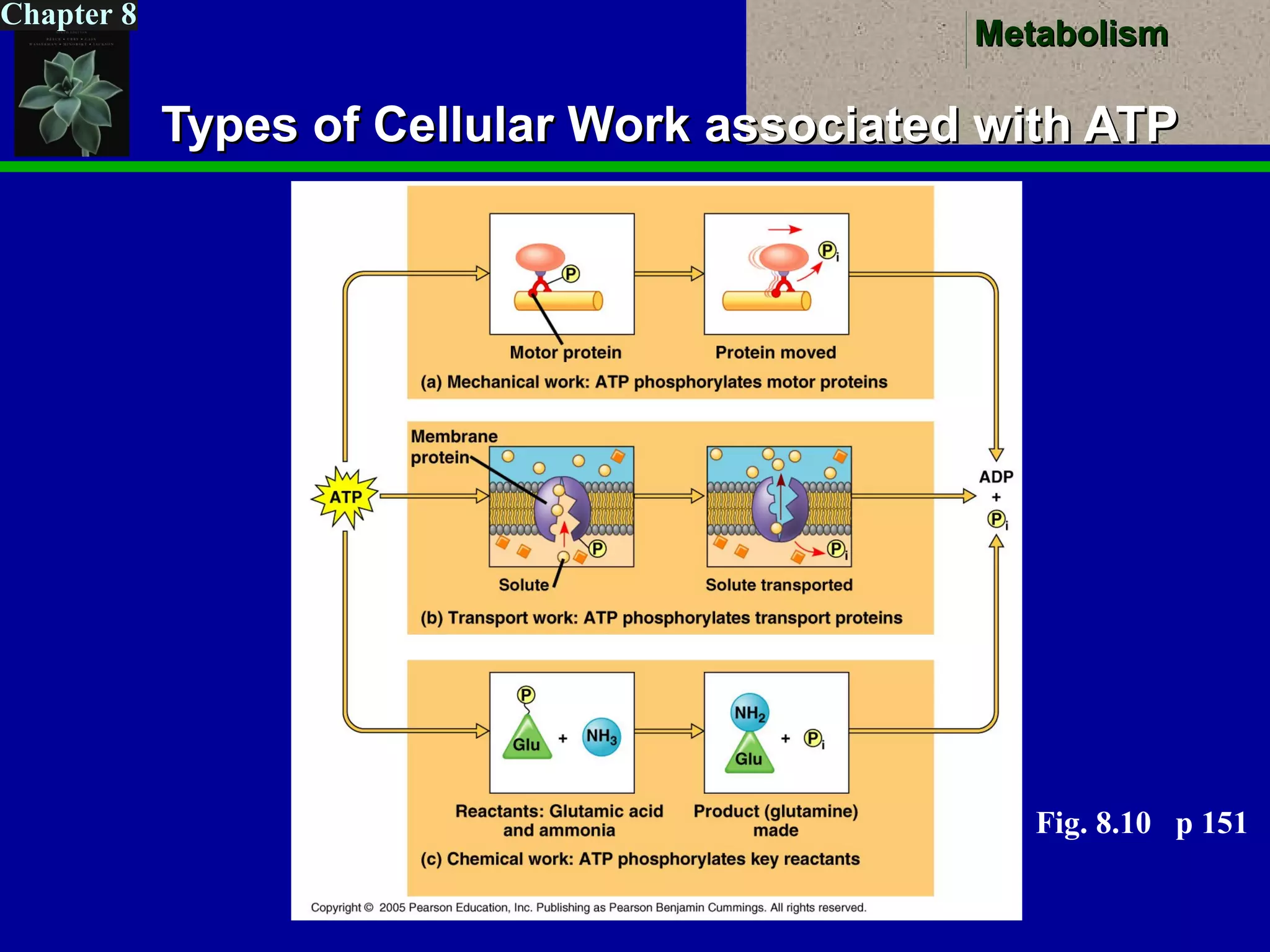
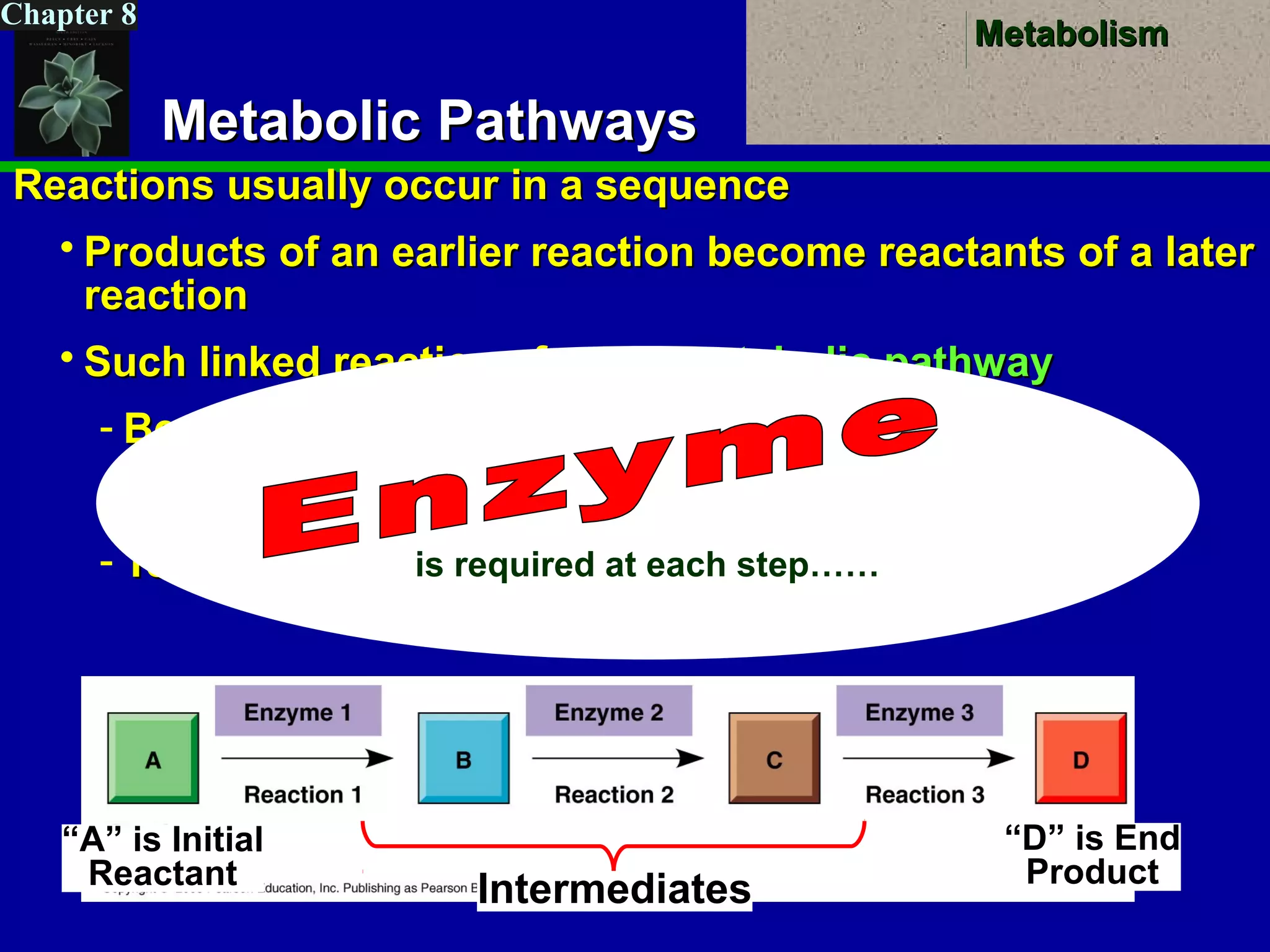
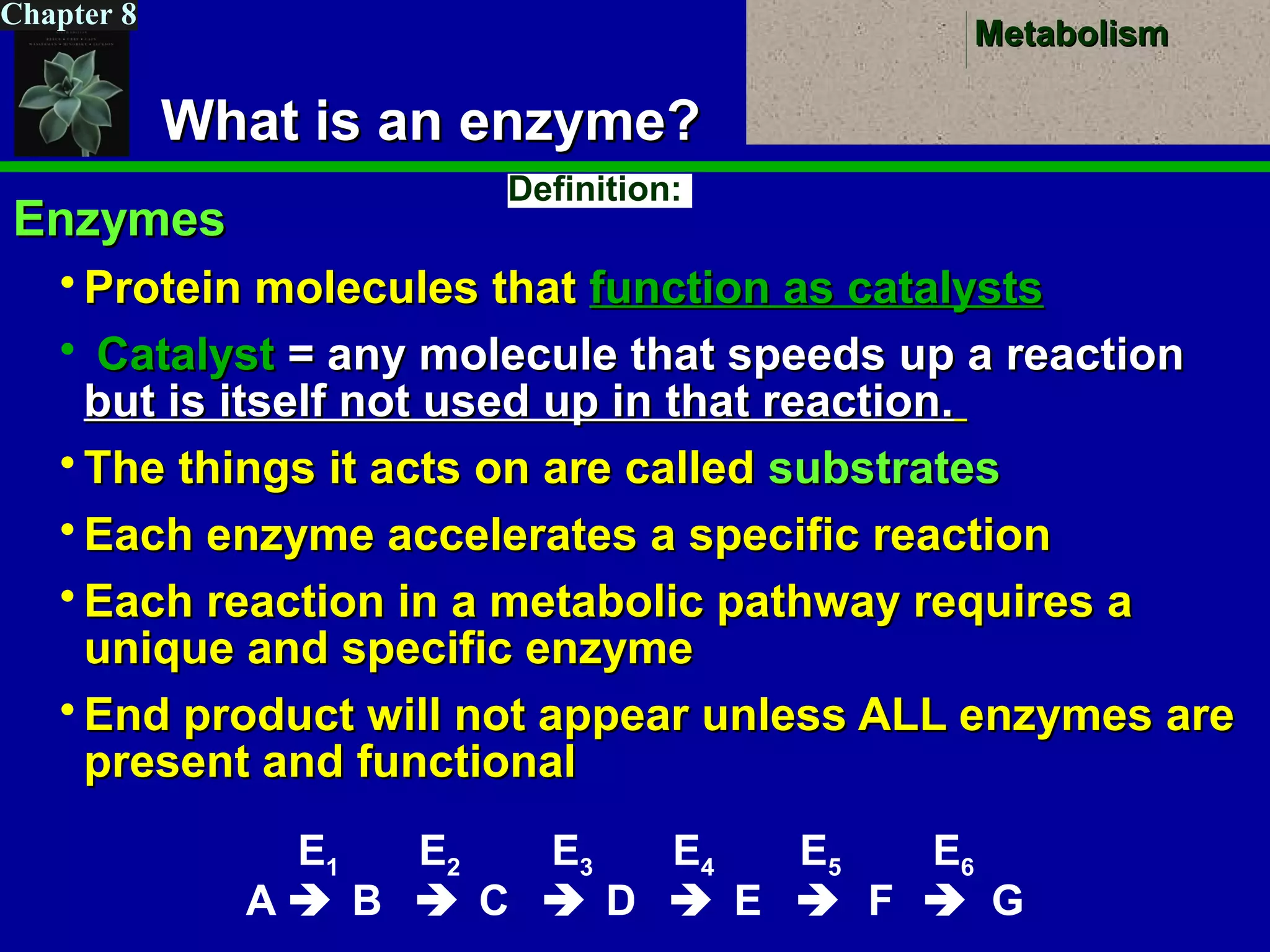
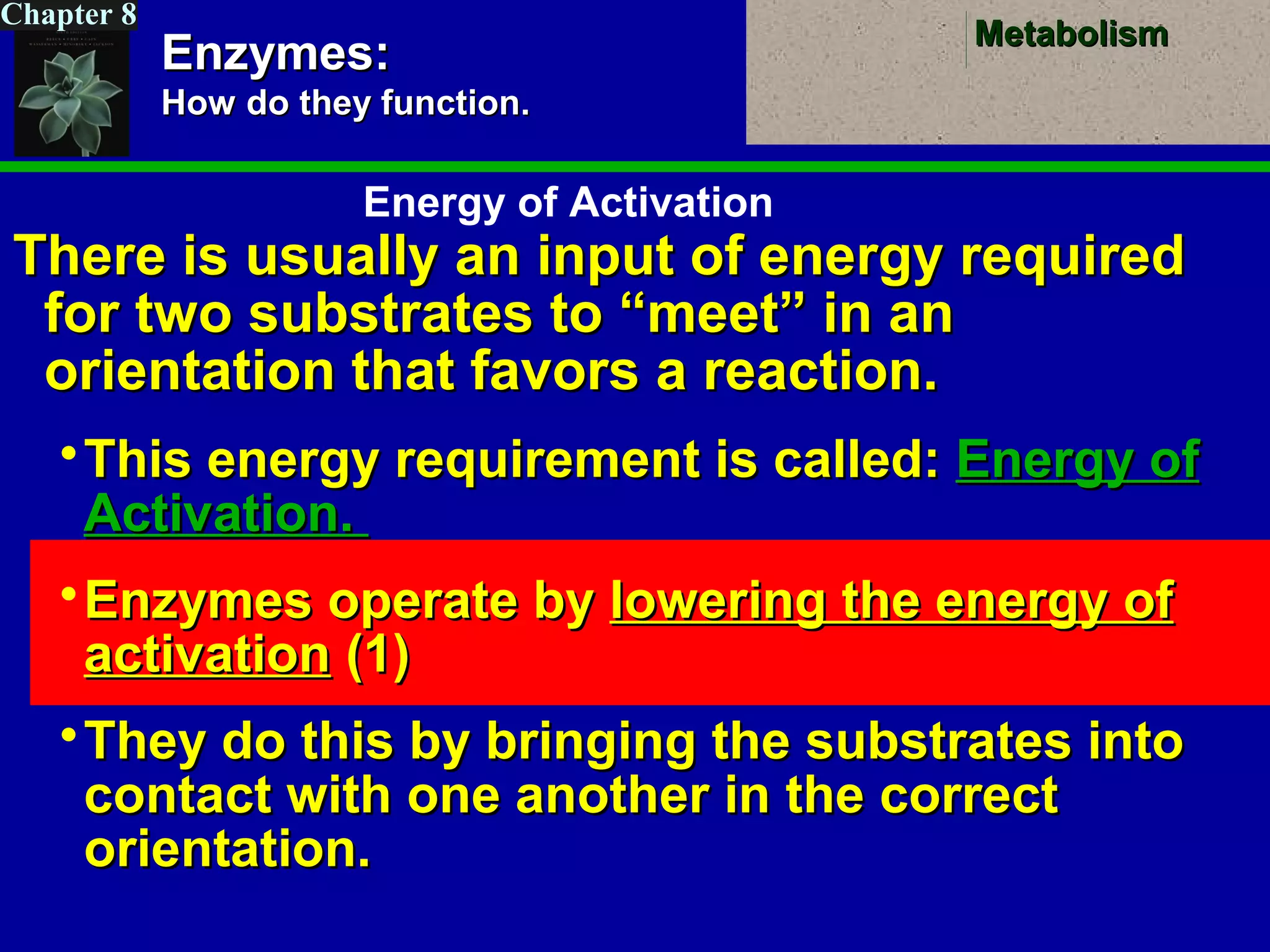
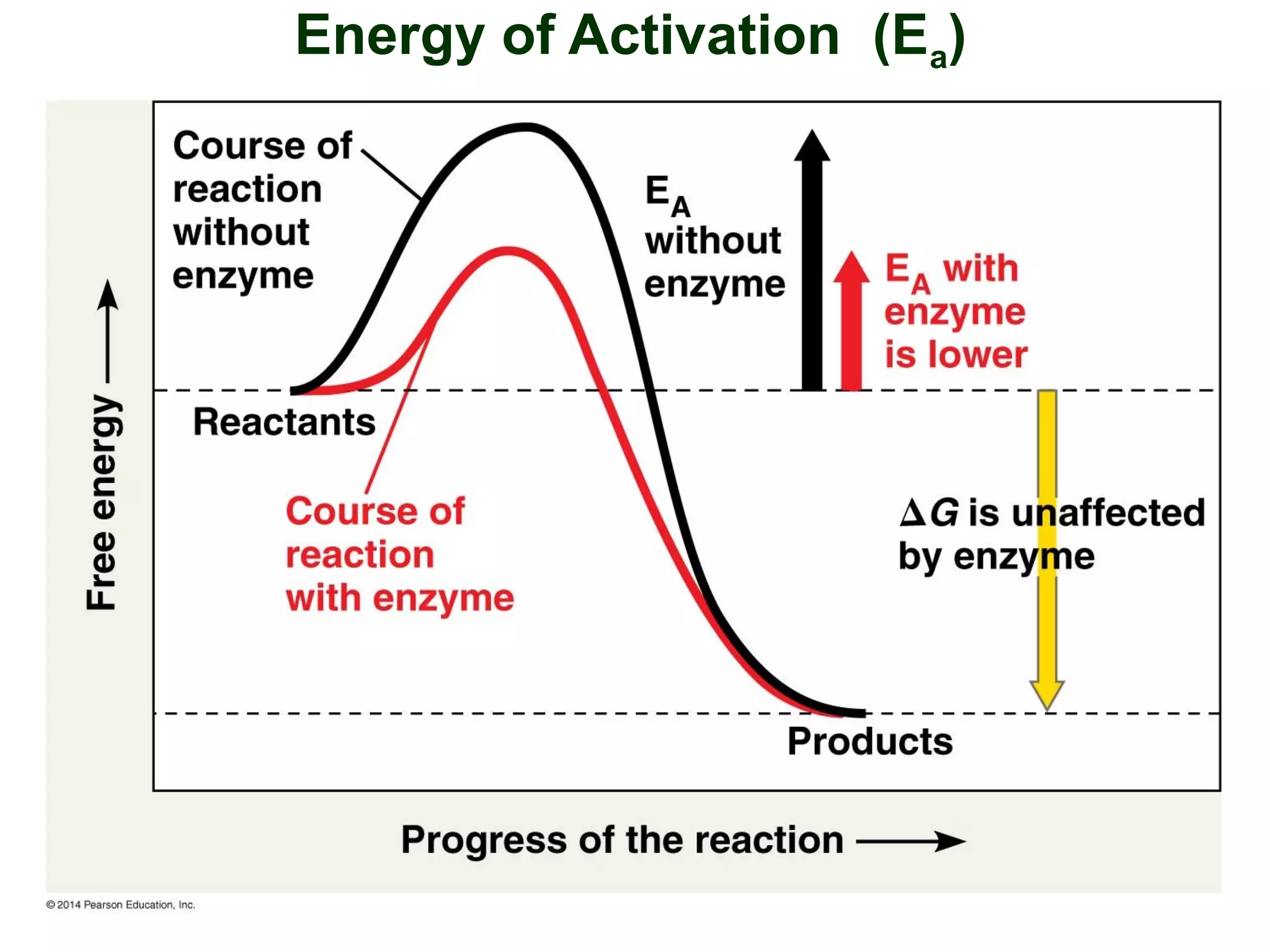
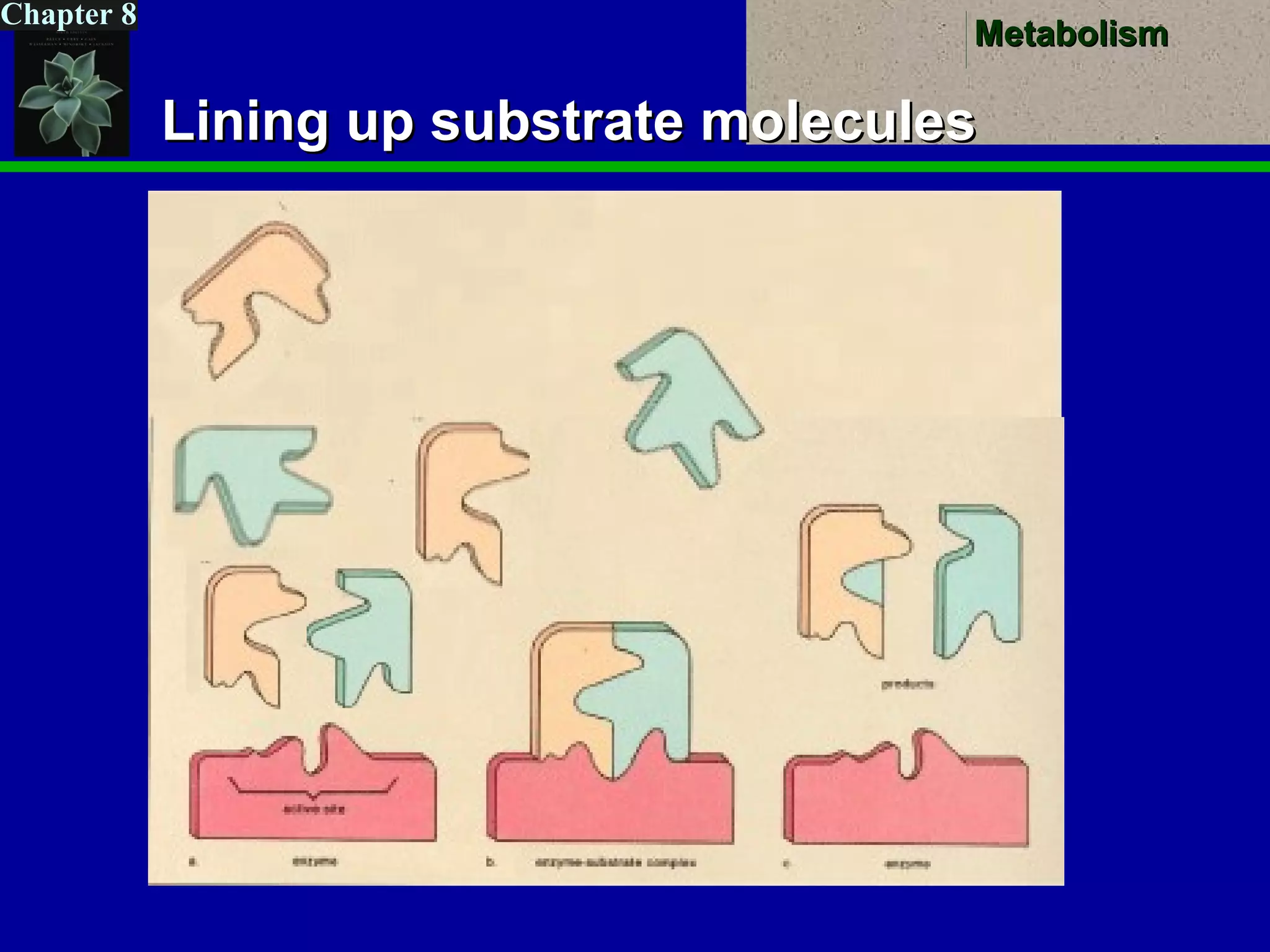
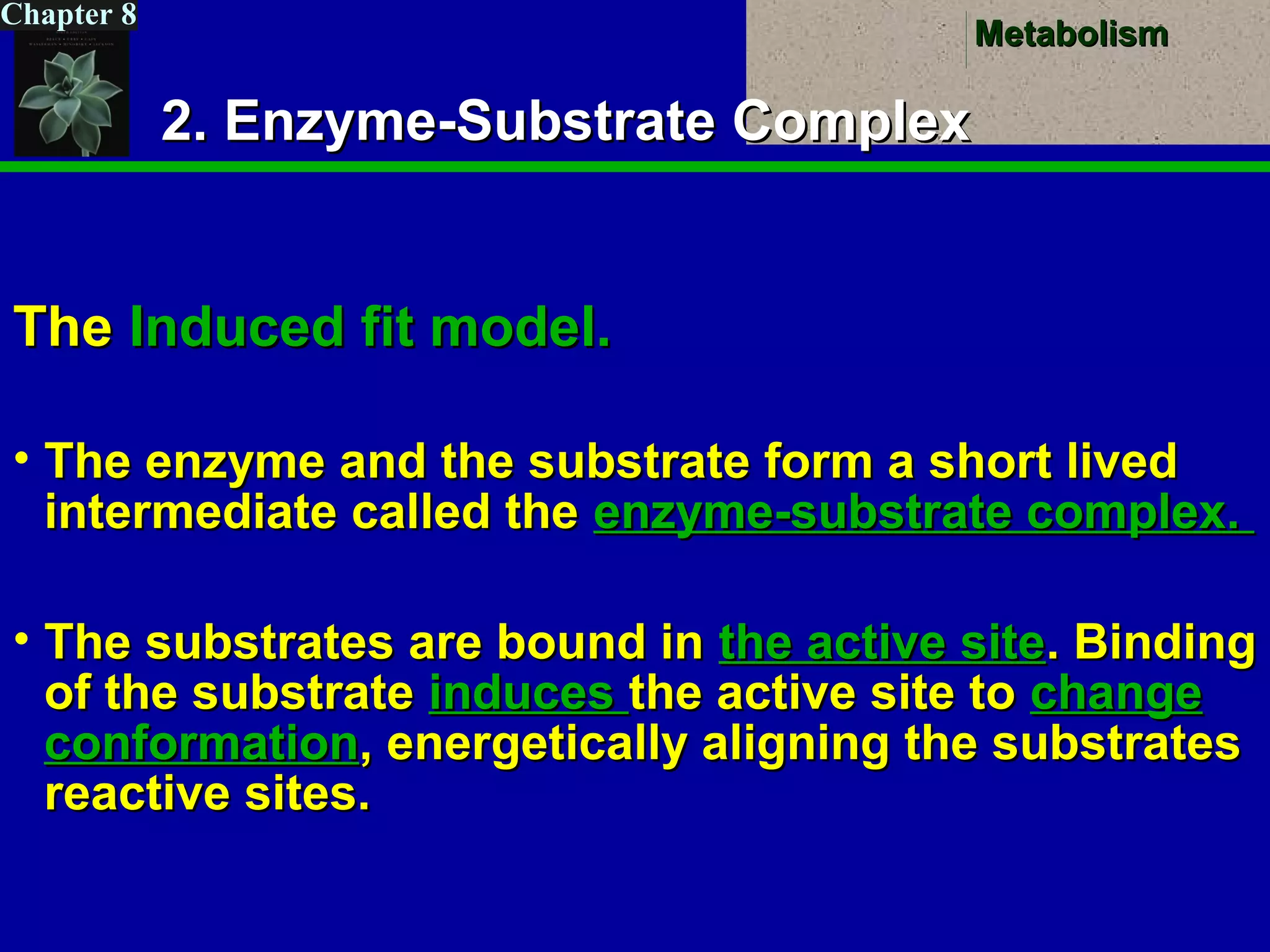
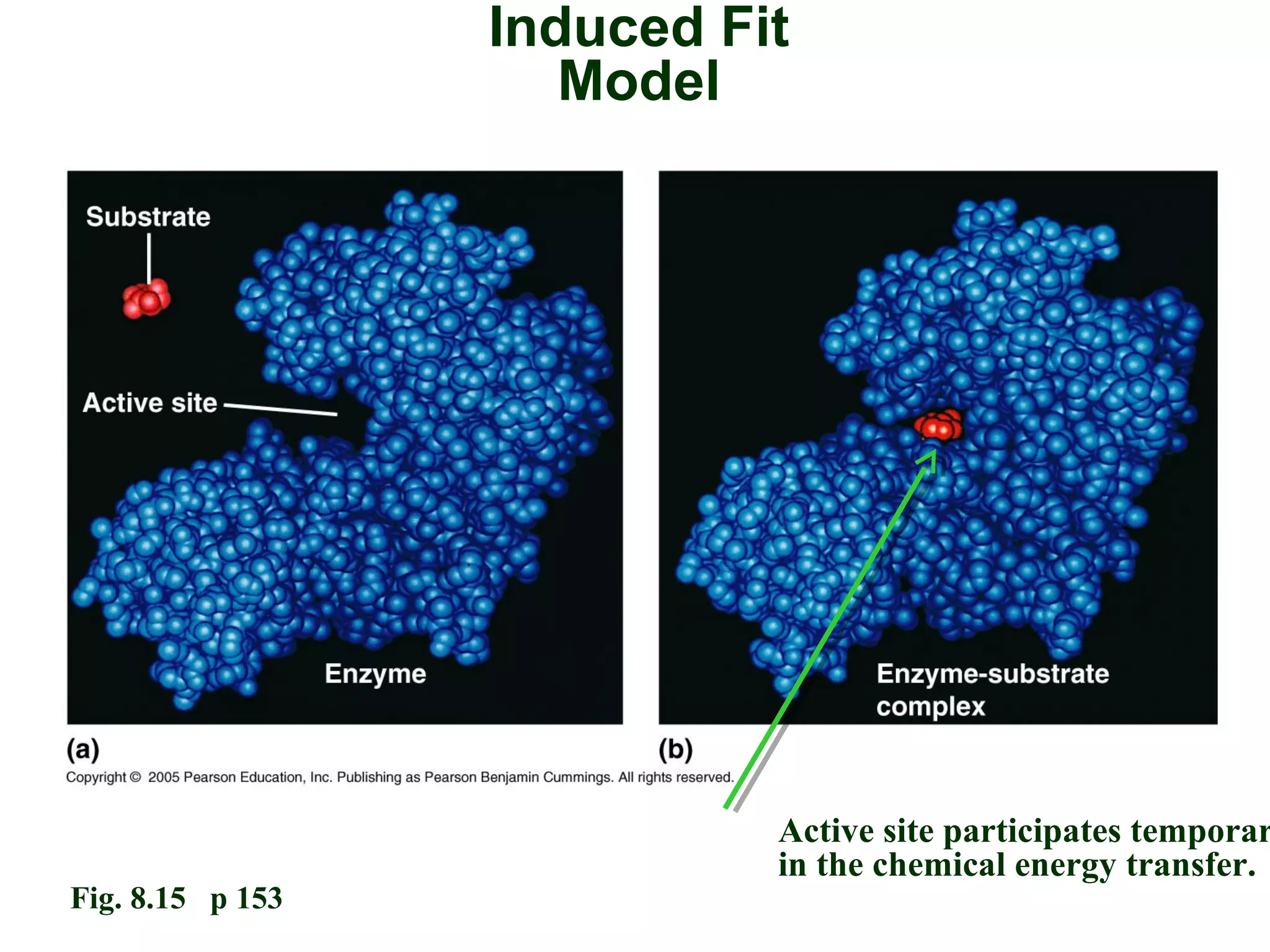
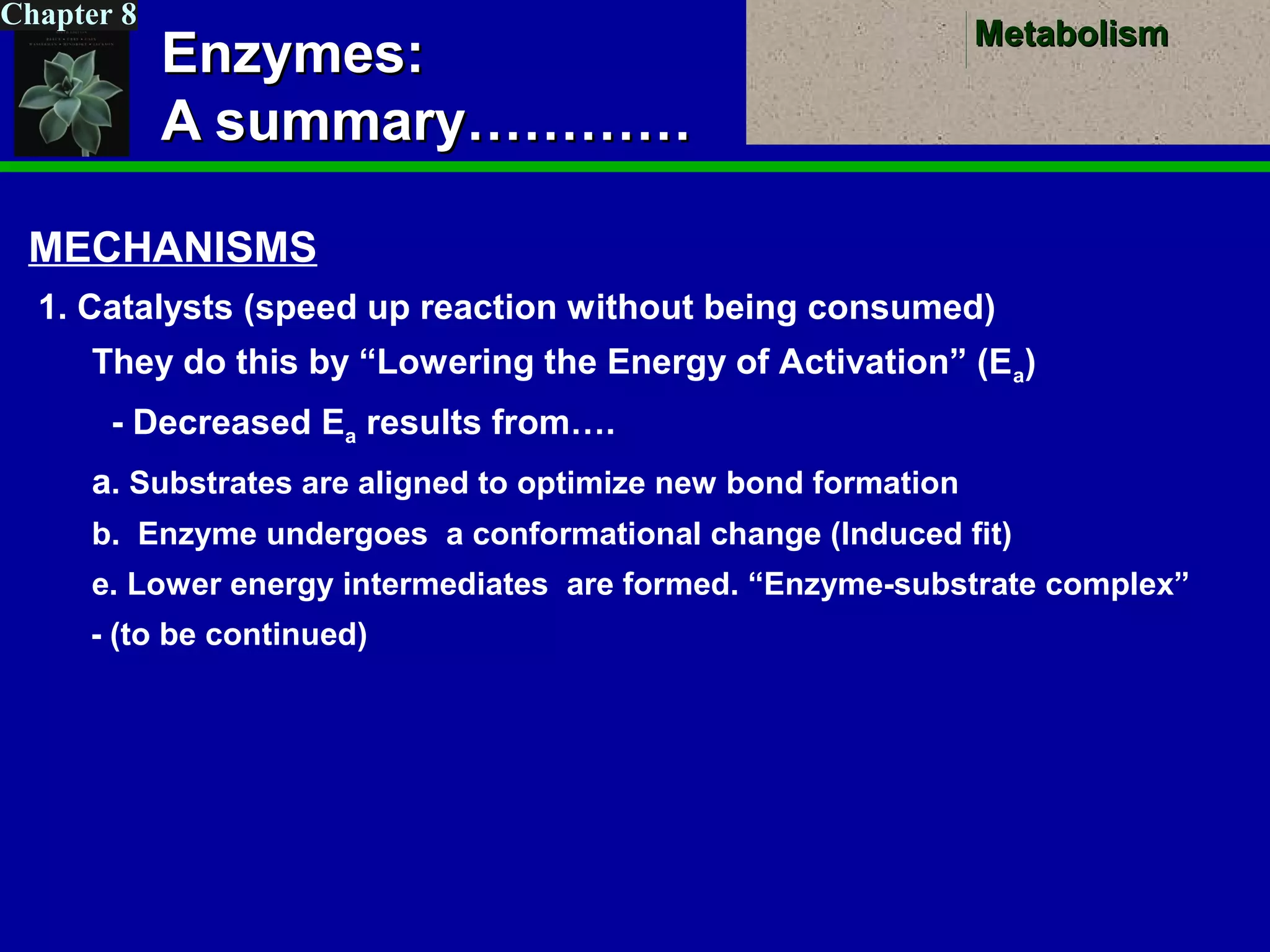
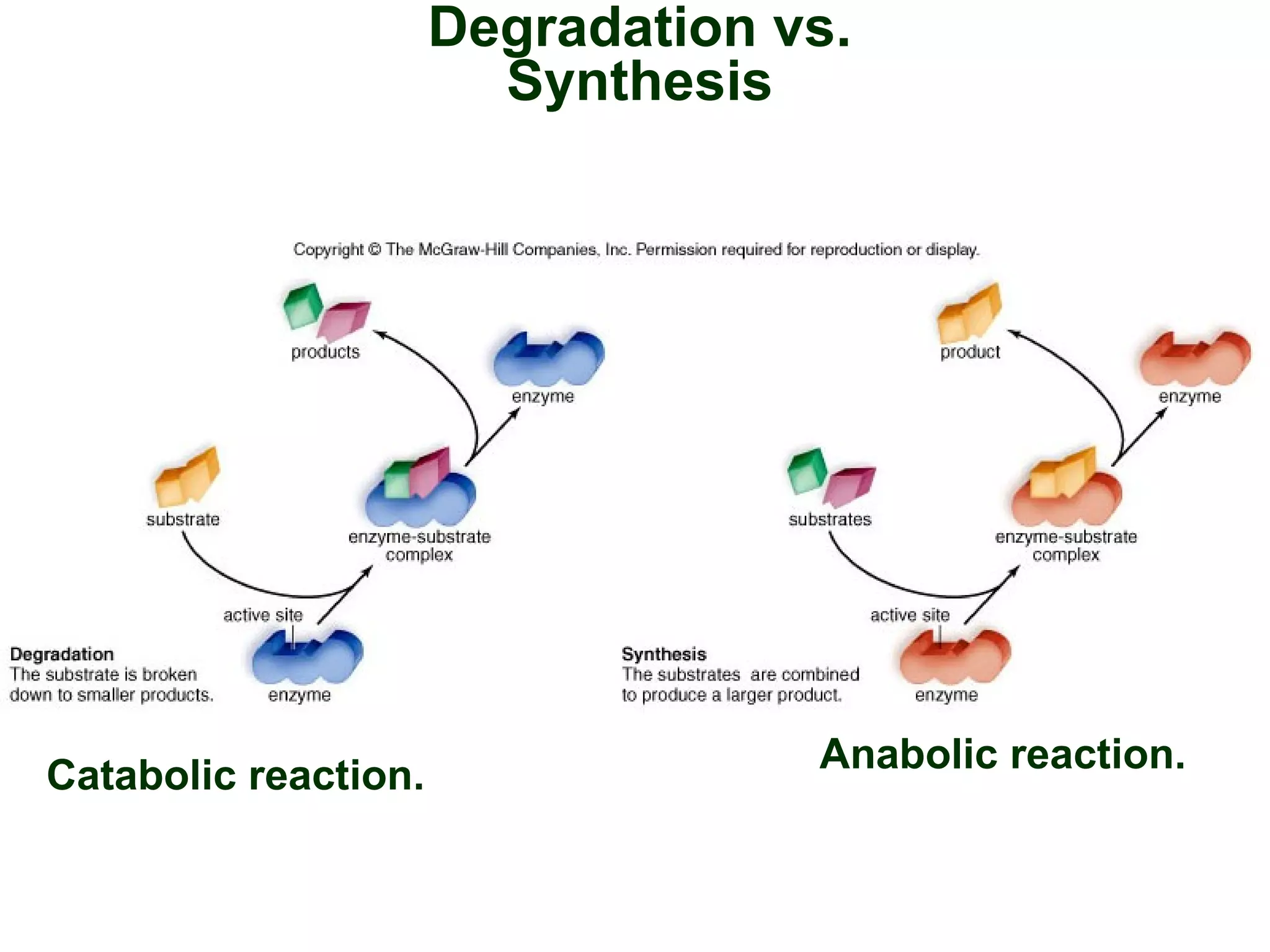
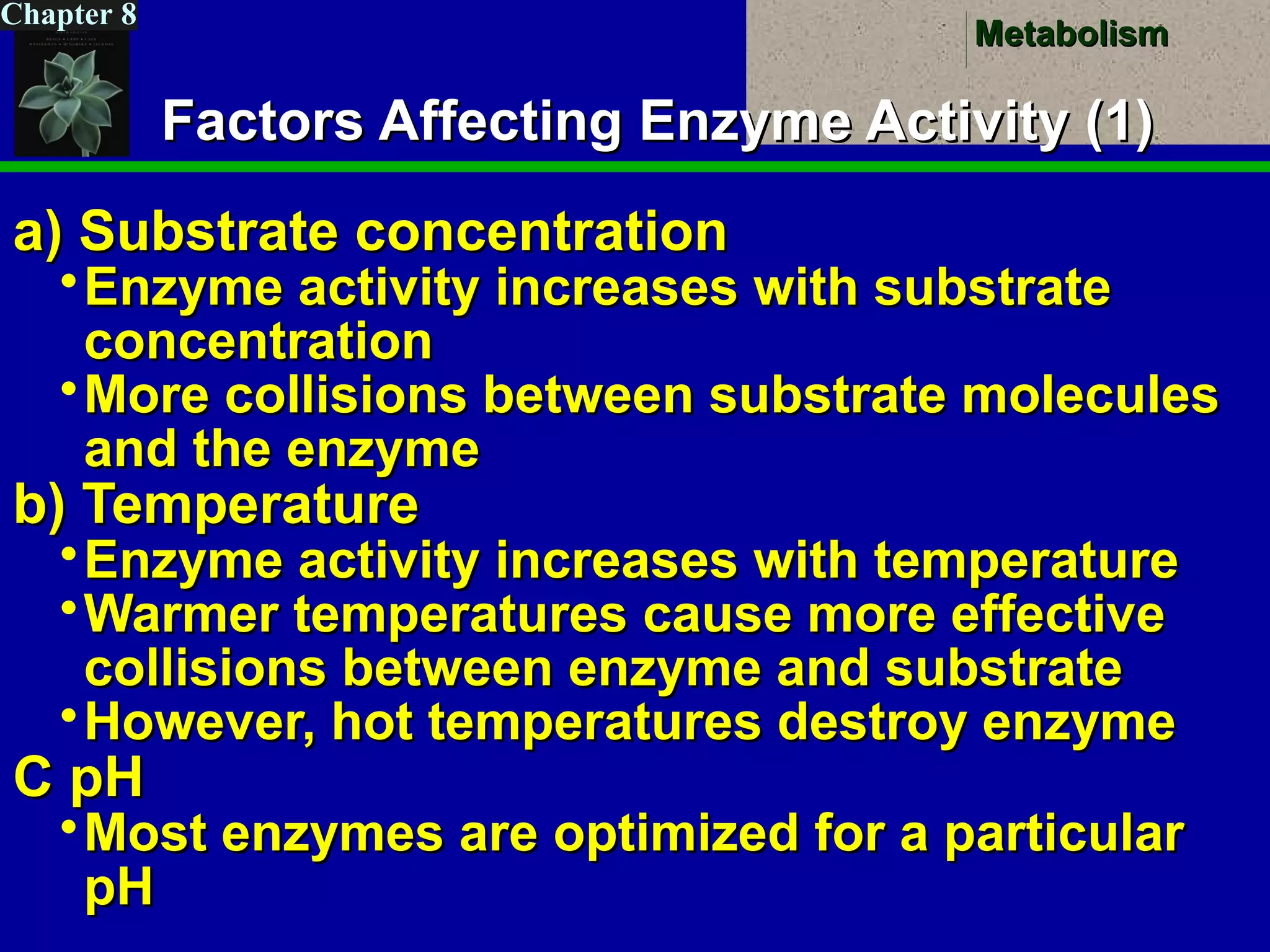
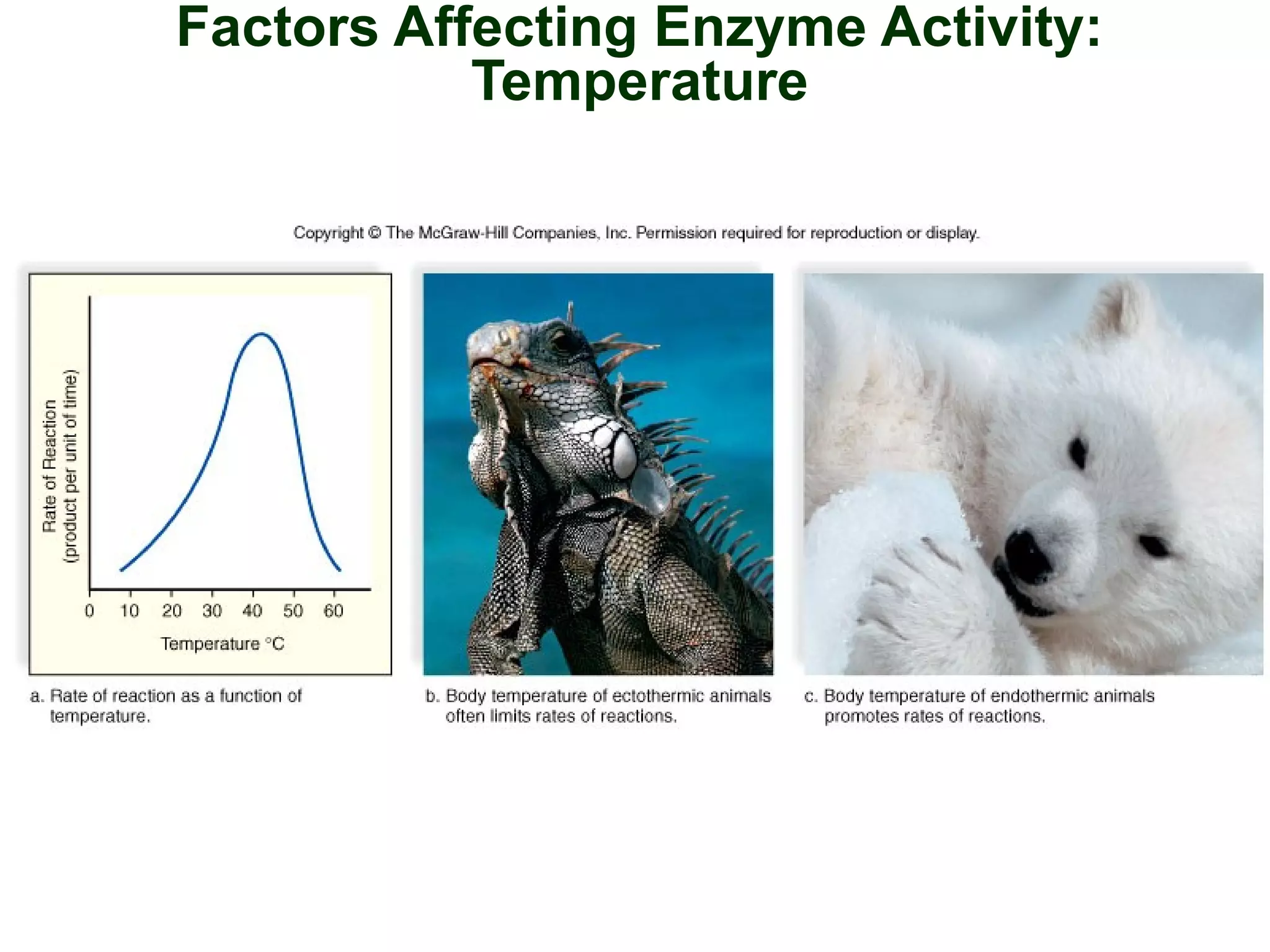
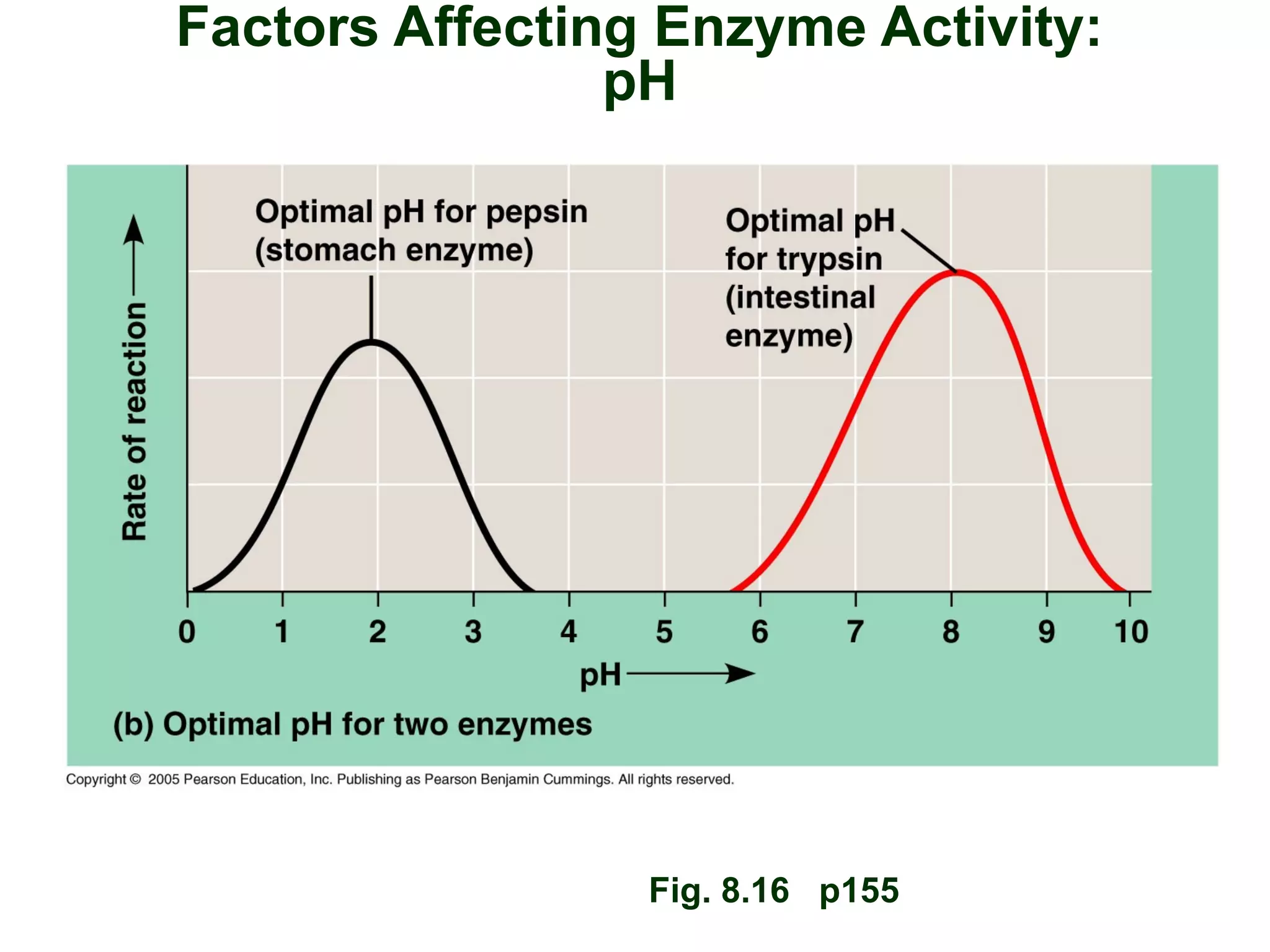
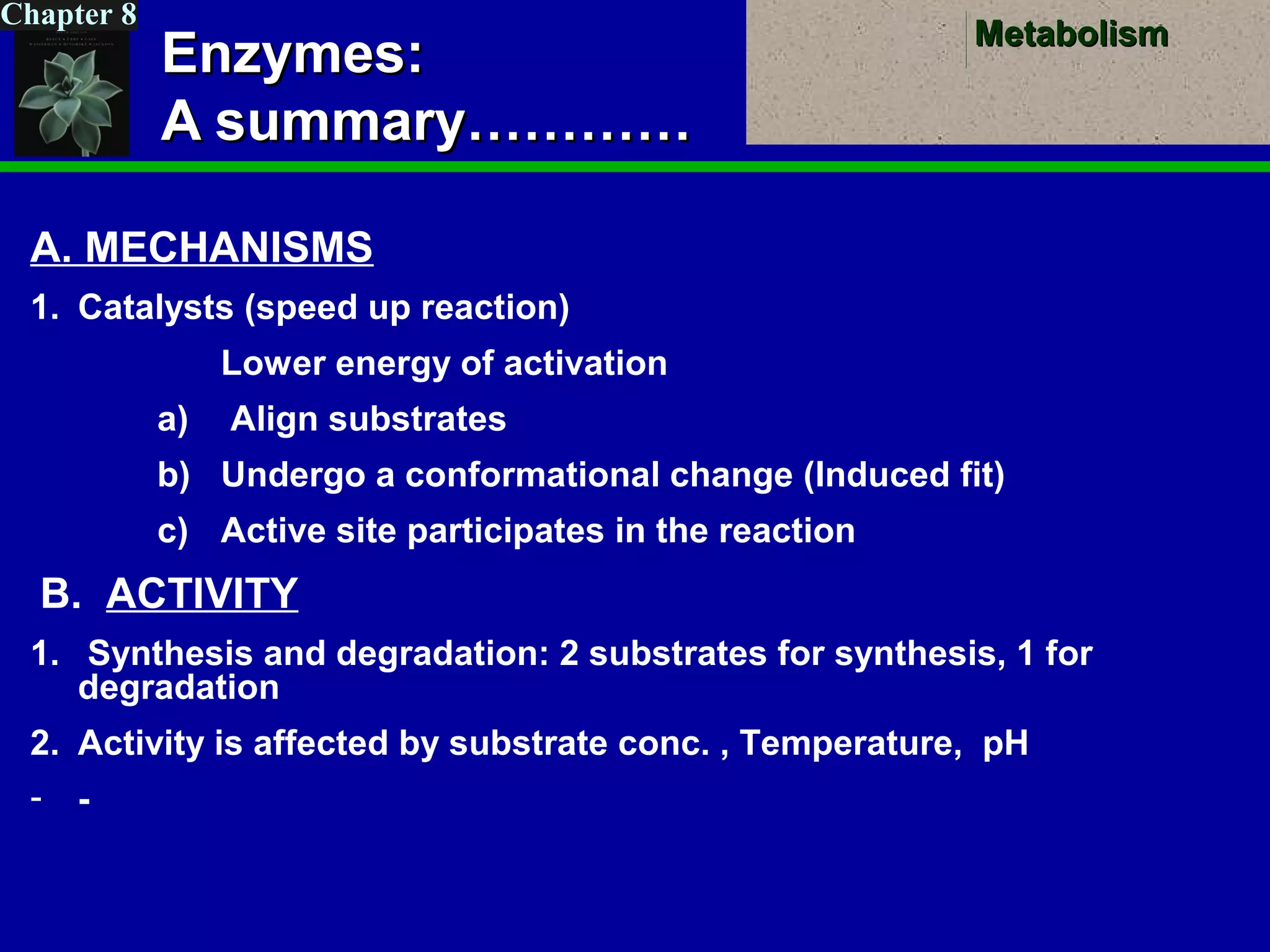
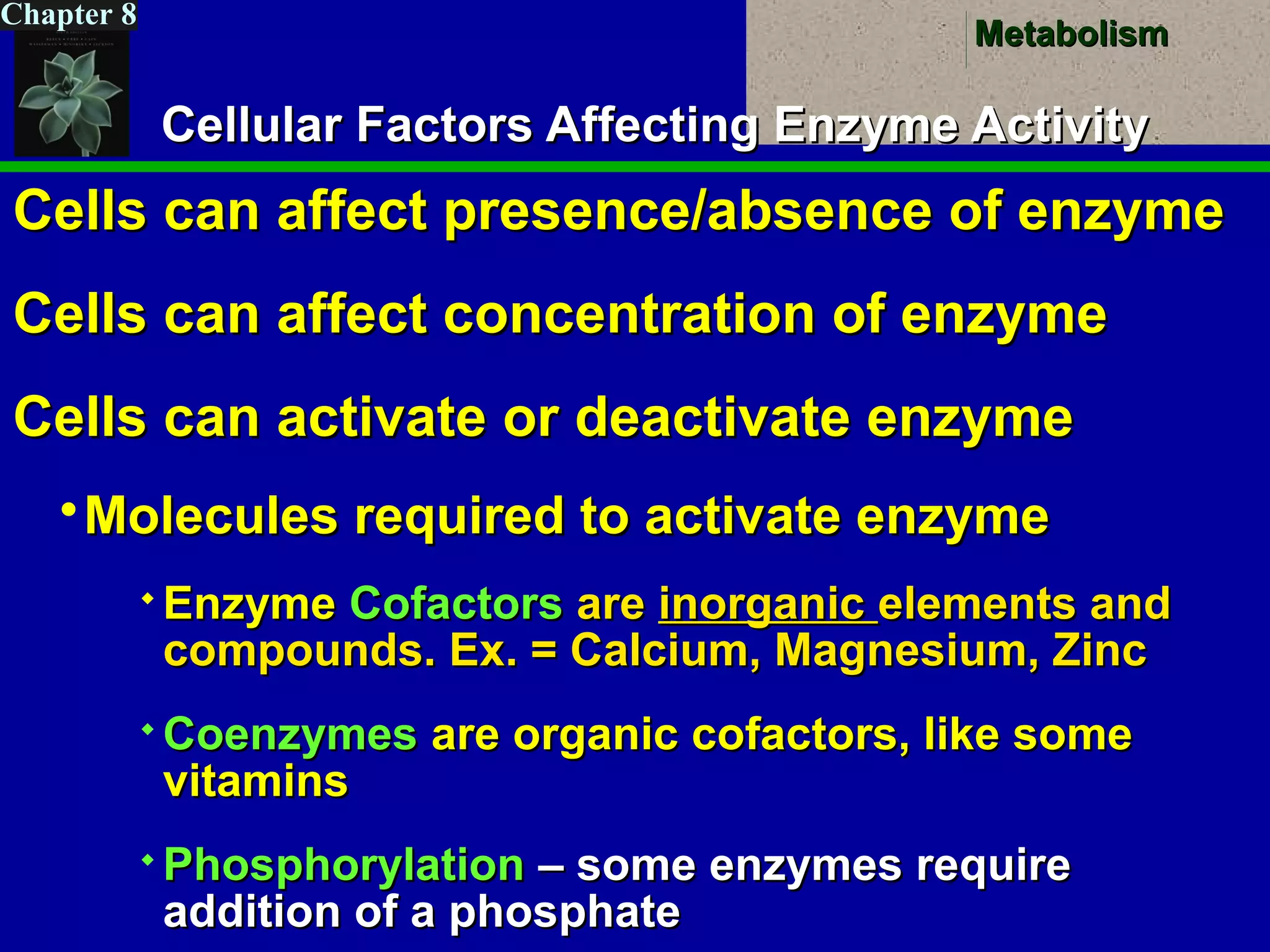
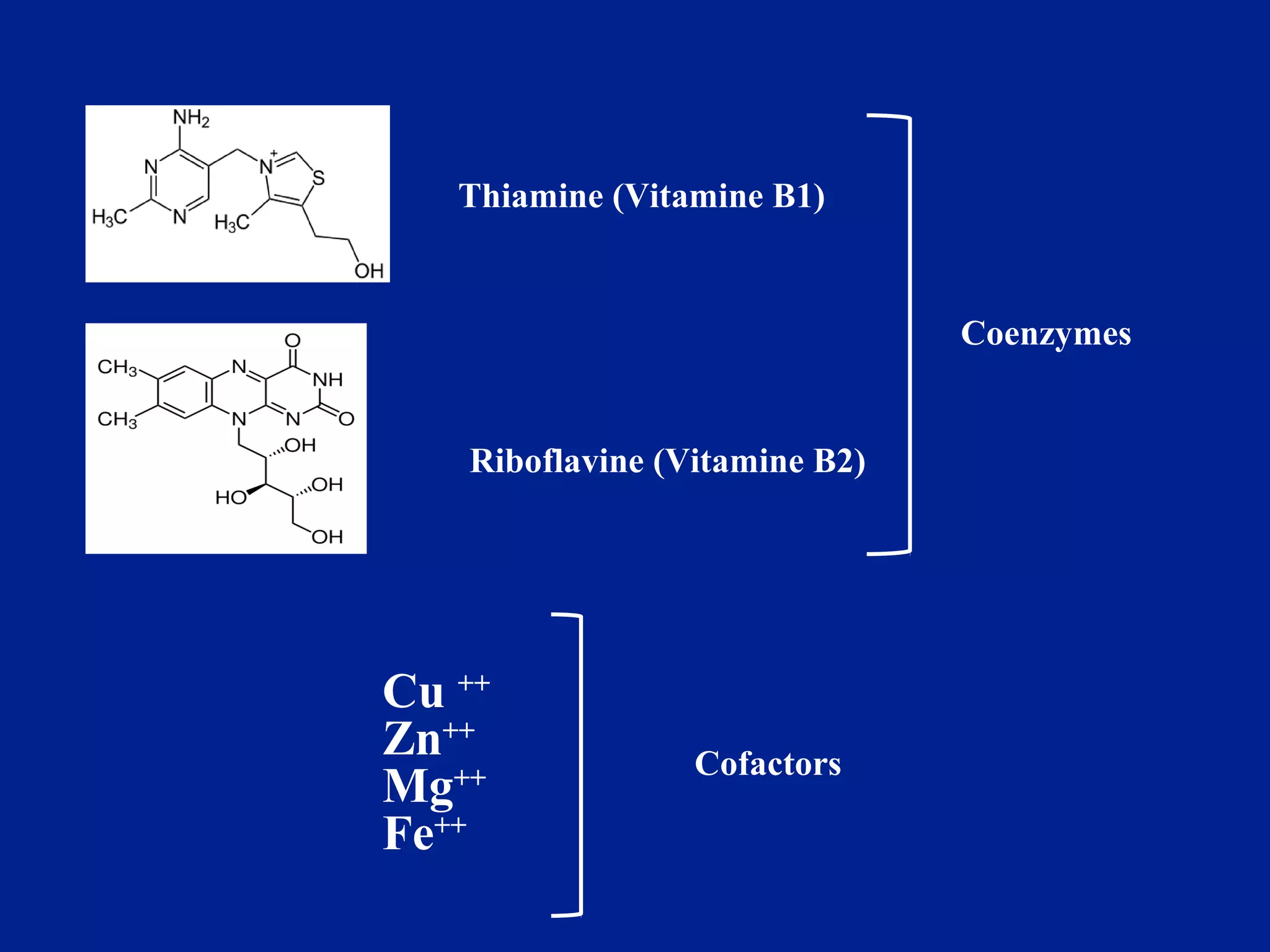
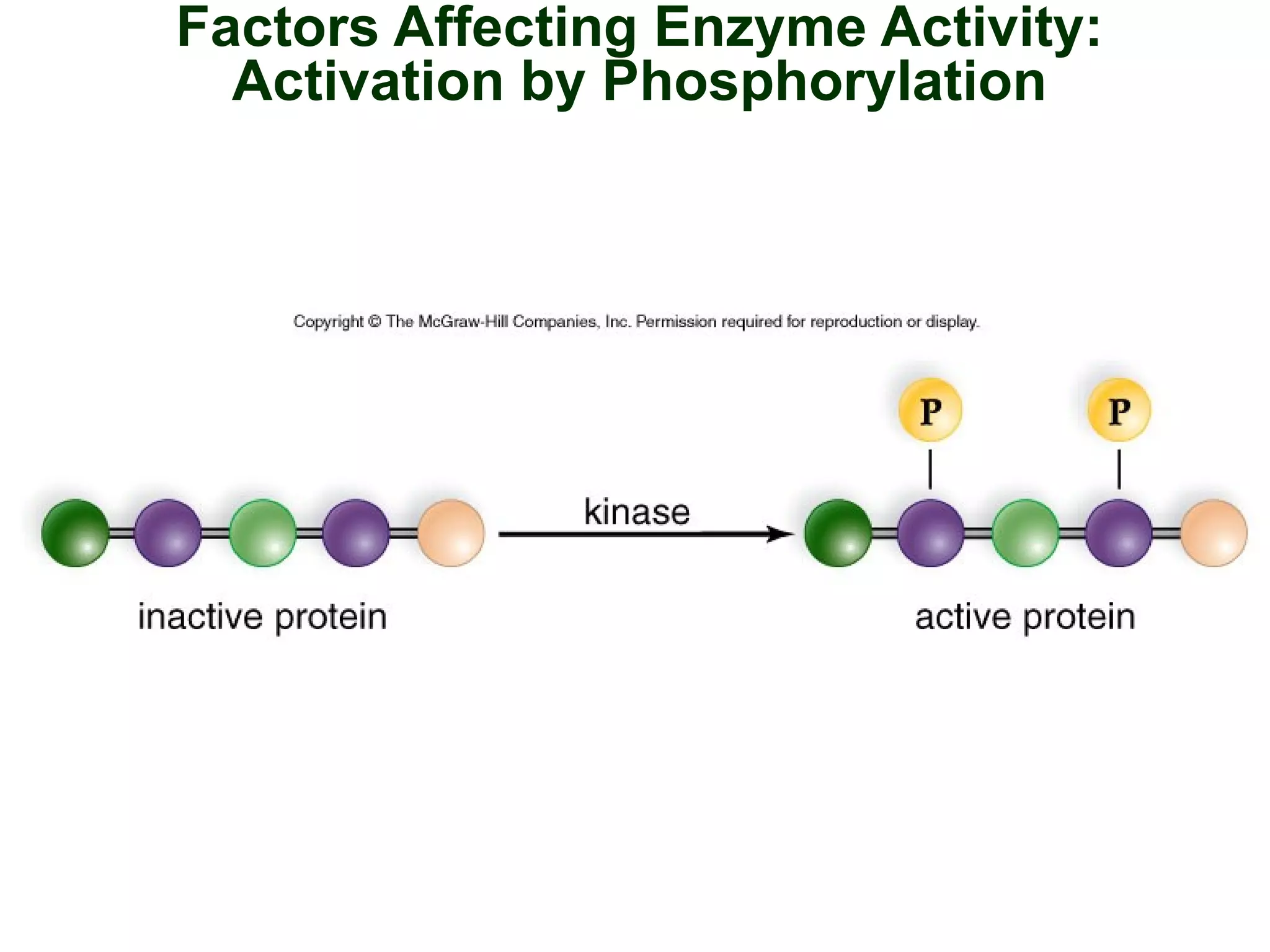
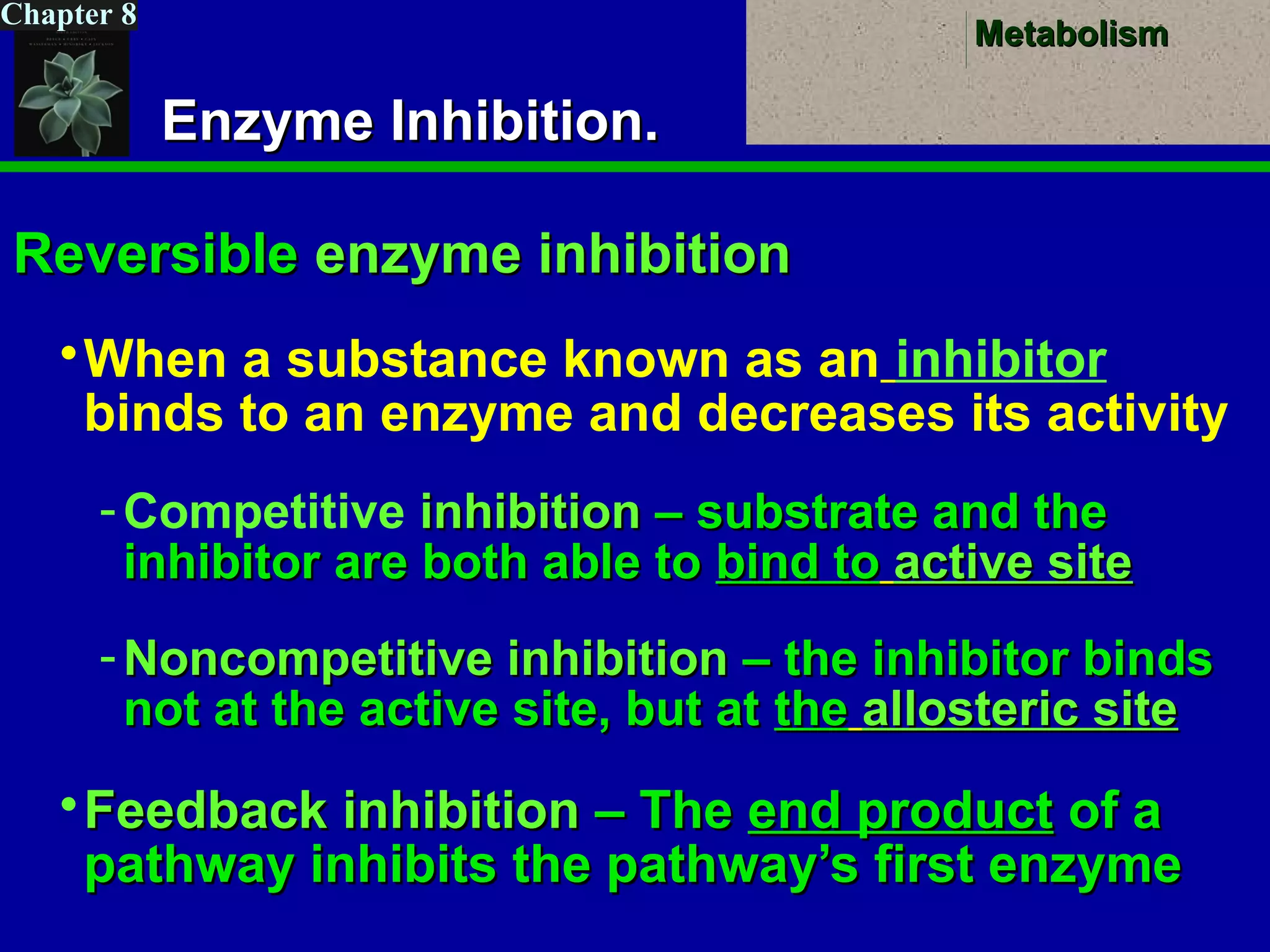
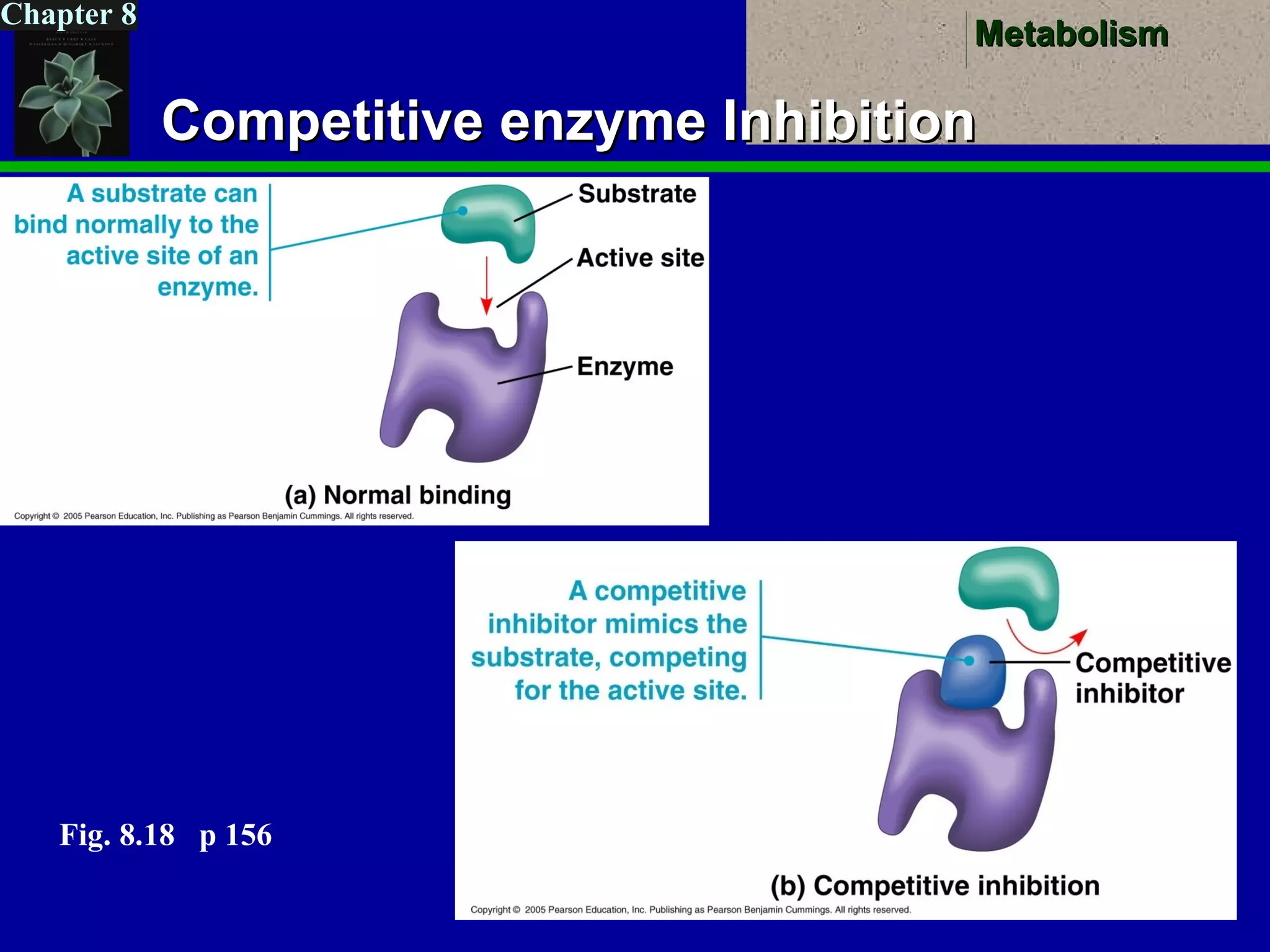
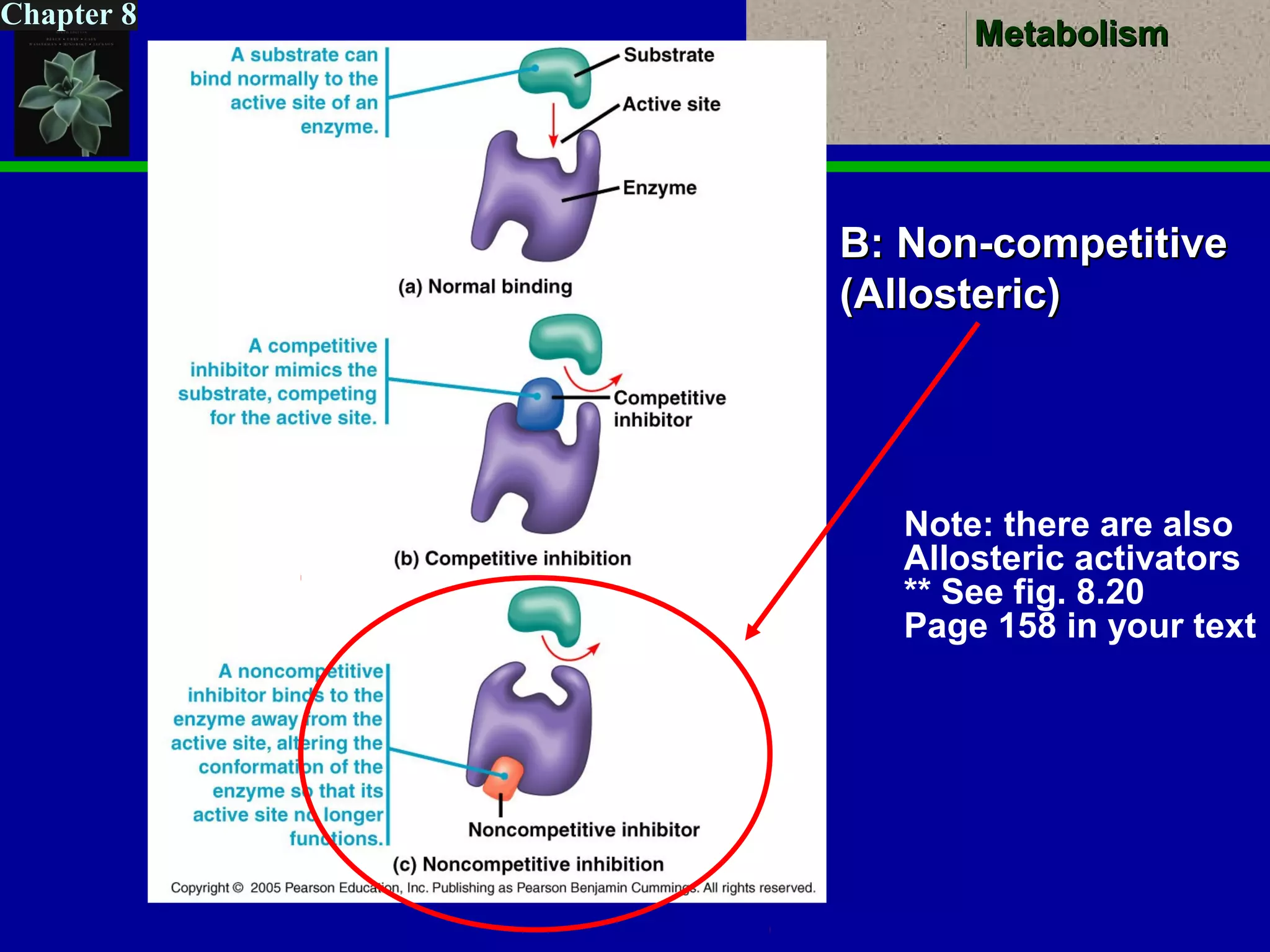
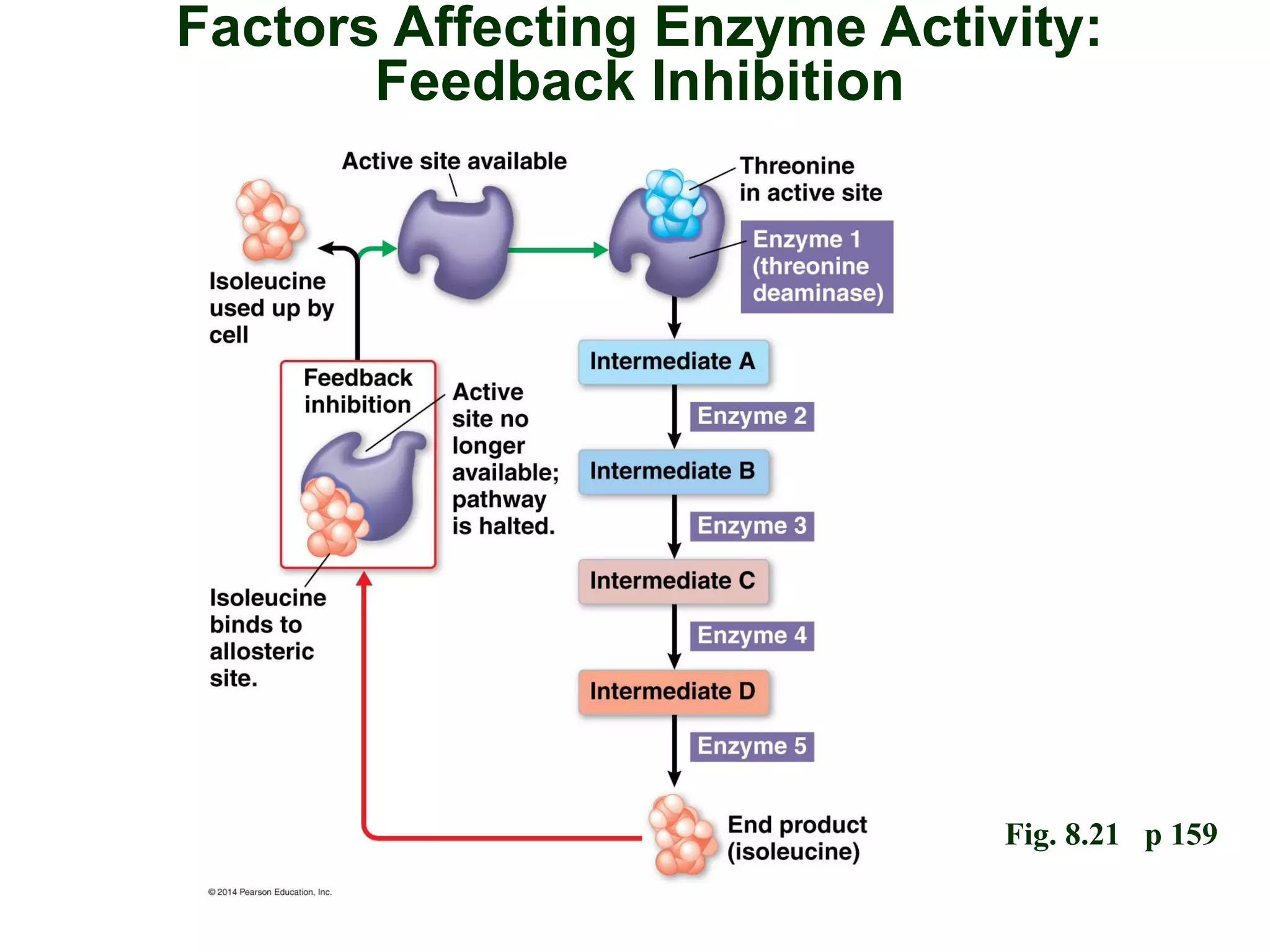
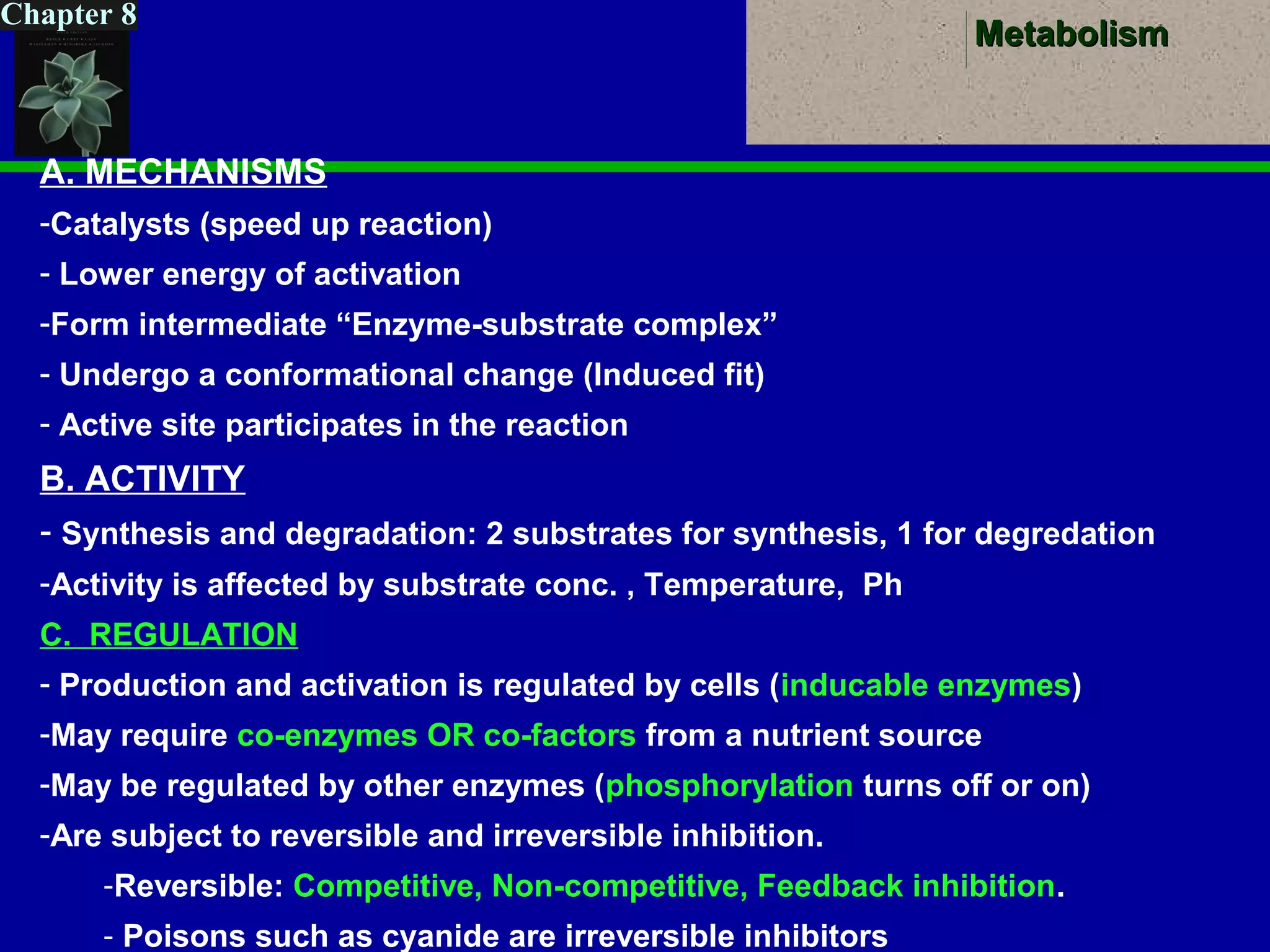
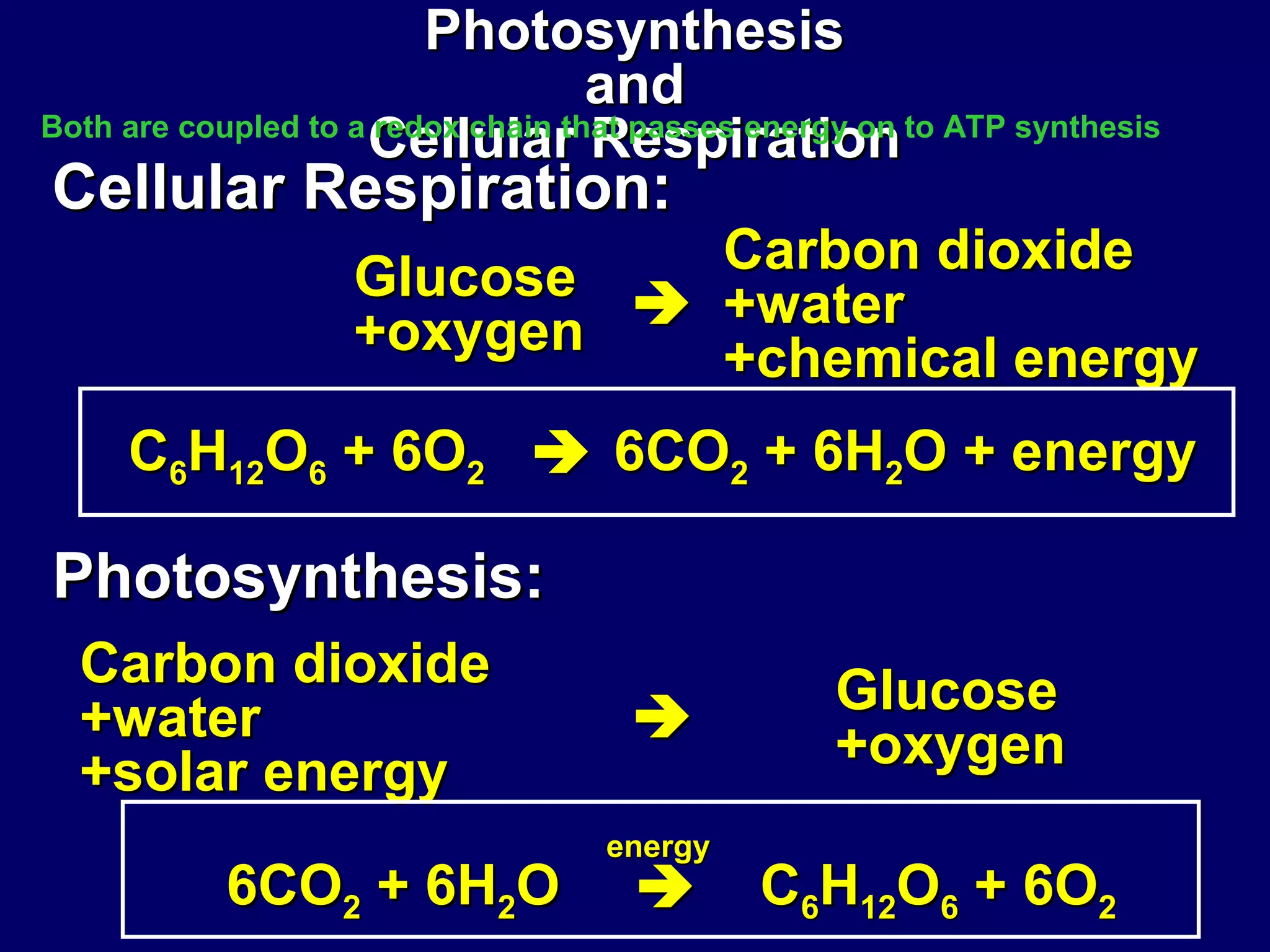
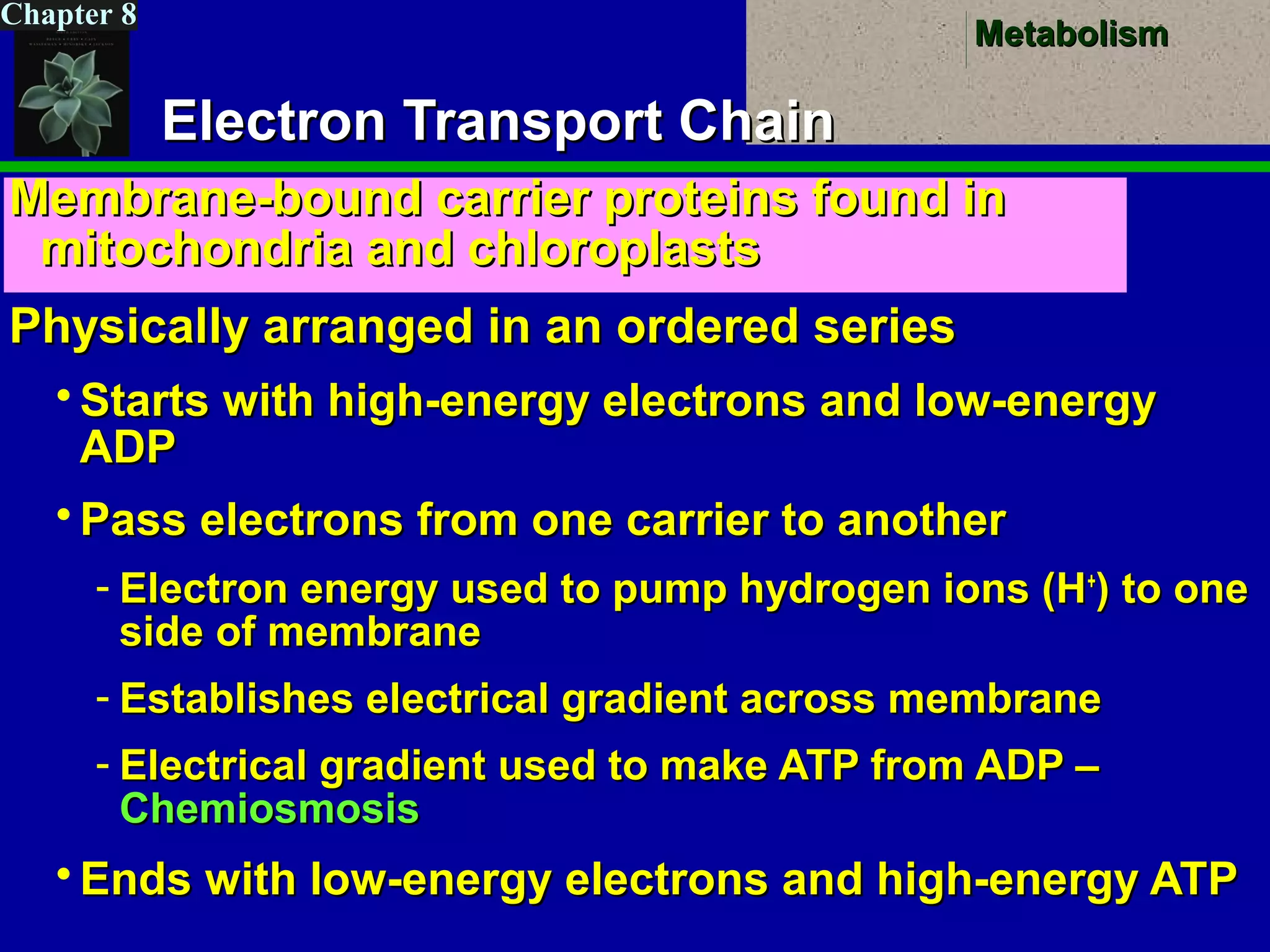
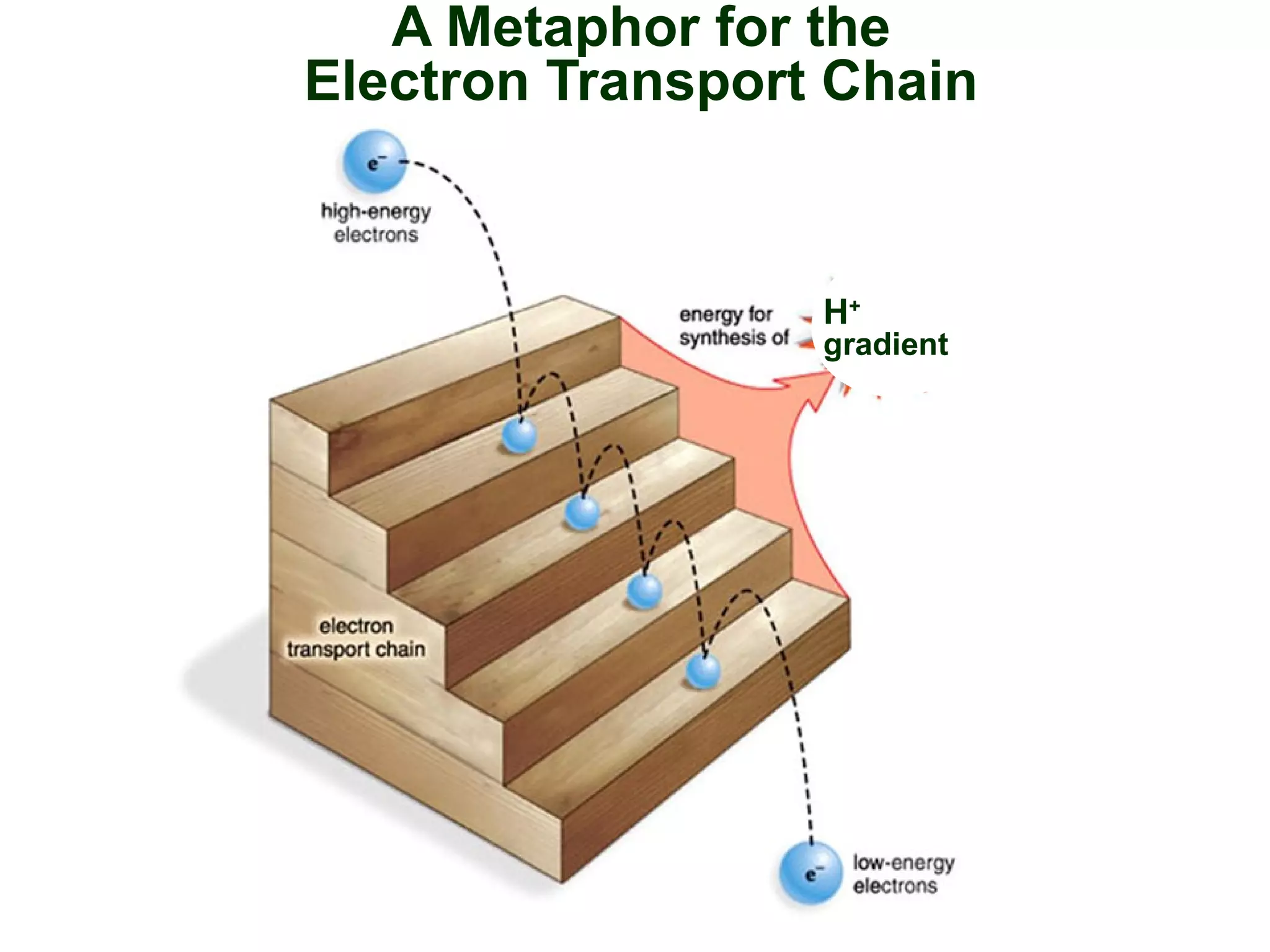
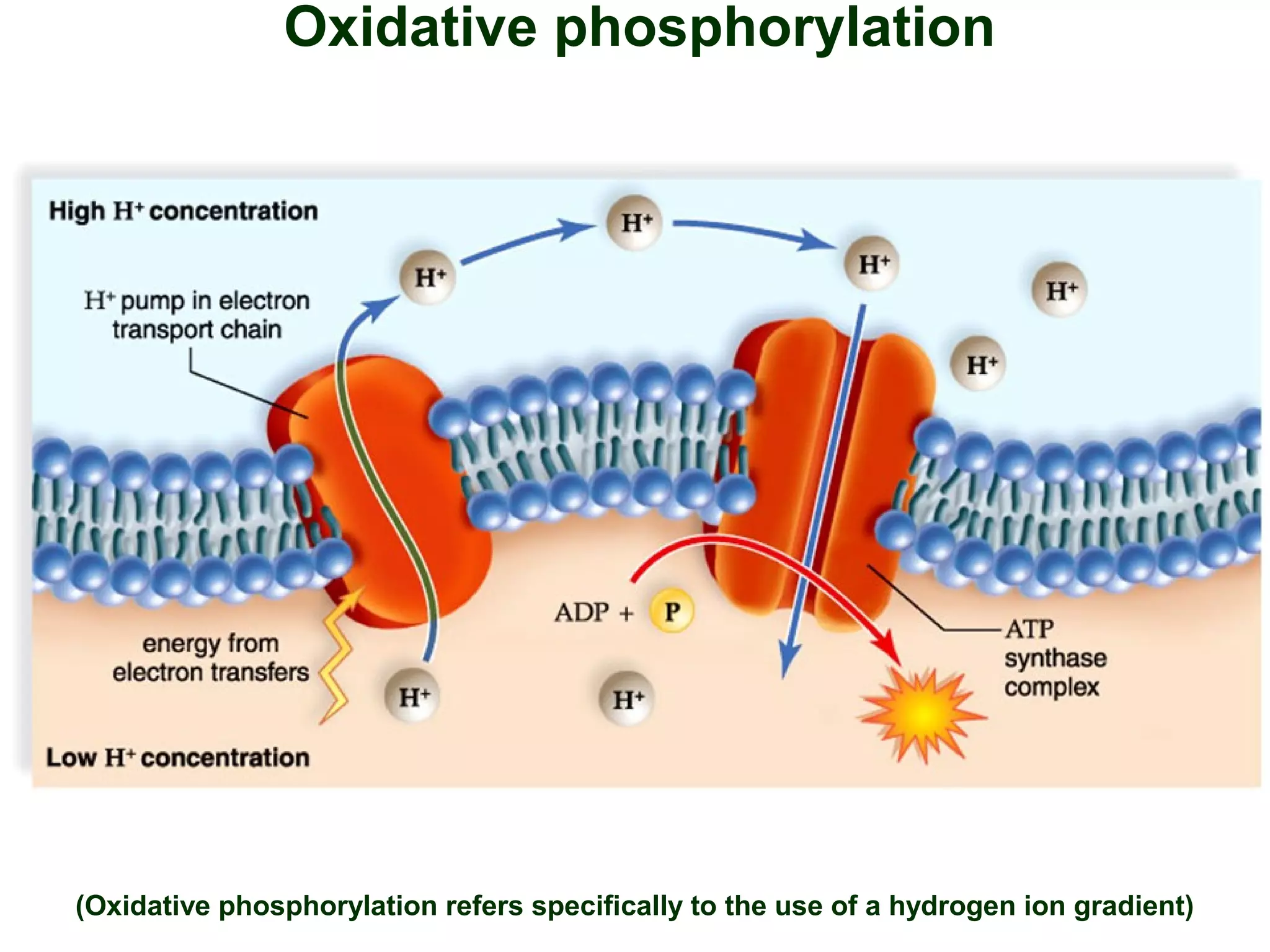
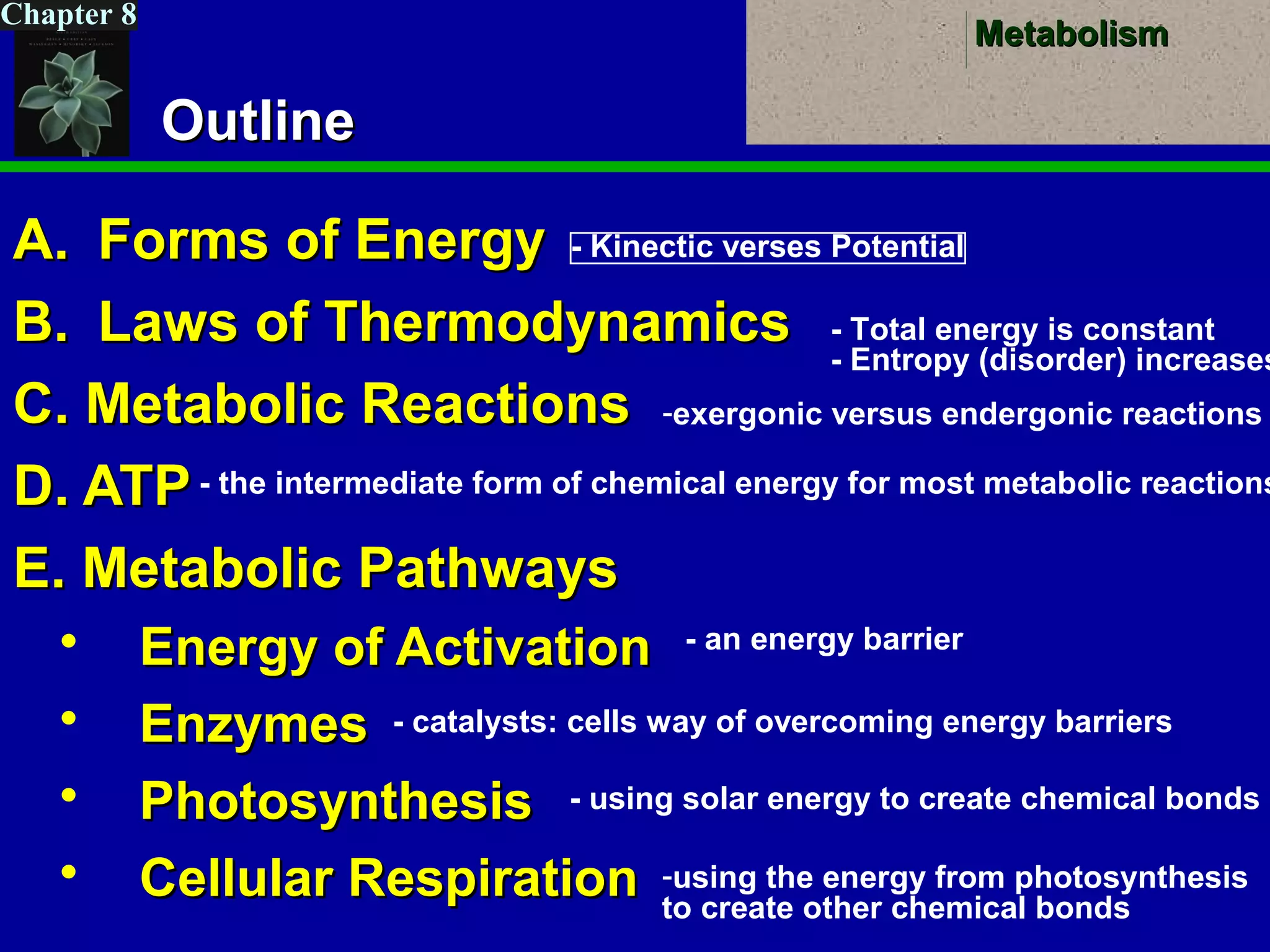
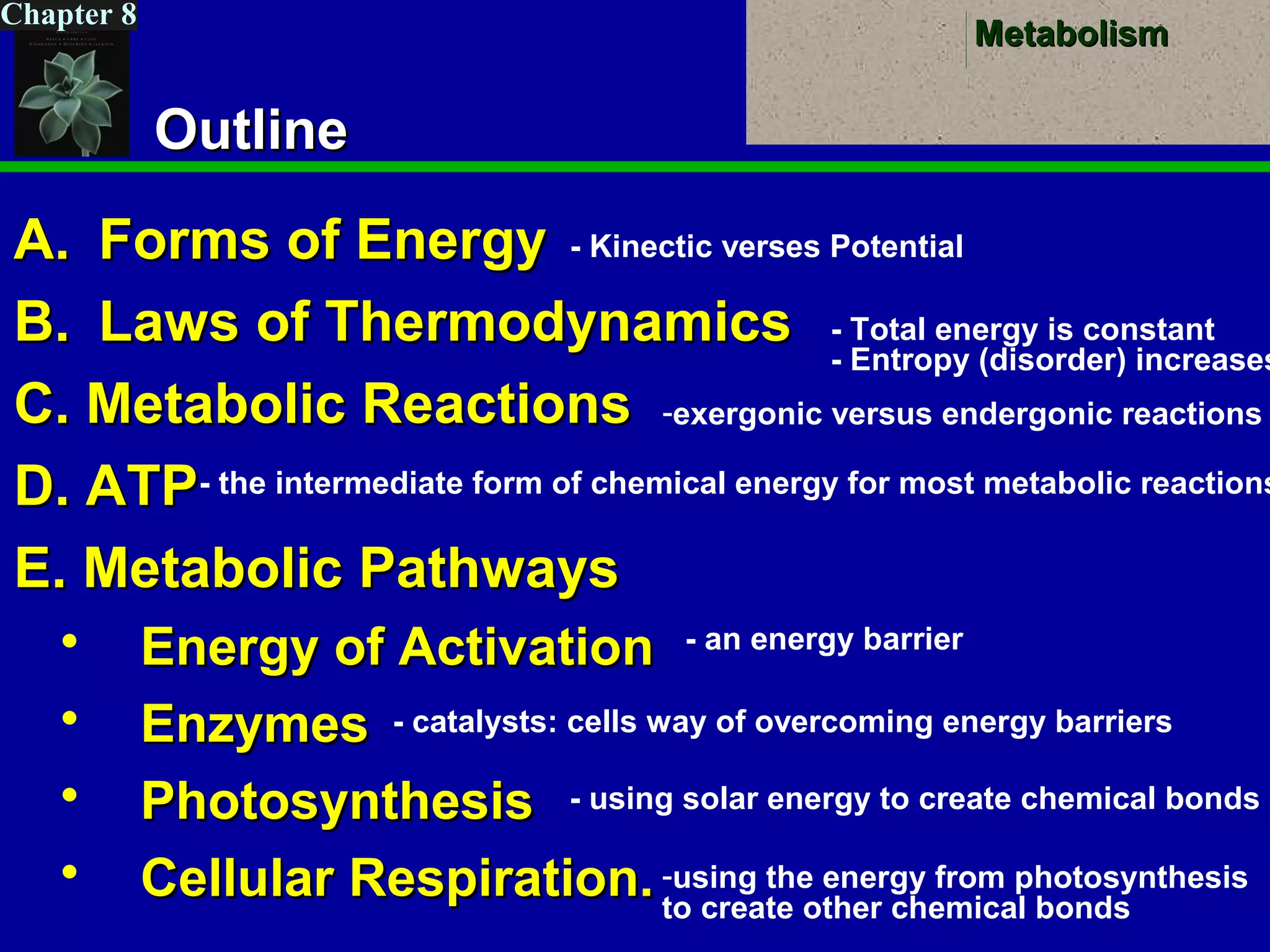
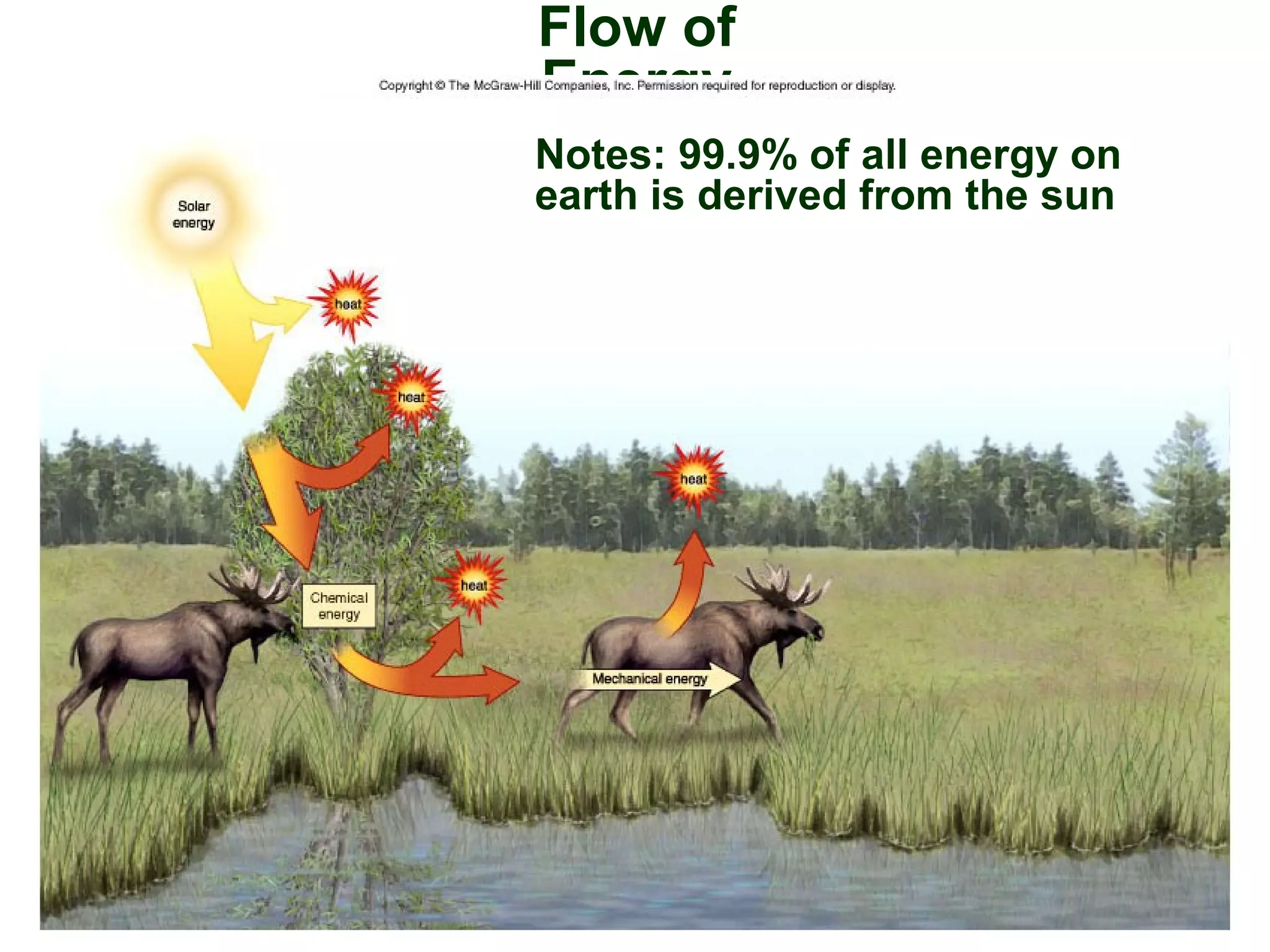
This document provides an overview of metabolism and metabolic reactions. It begins by defining the key concepts of kinetic and potential energy. It then explains the laws of thermodynamics and how they apply to biological systems. Specifically, it states that energy cannot be created or destroyed, and that entropy increases with energy transfers. It introduces the idea of exergonic and endergonic reactions, and how cells harness energy from exergonic reactions through ATP. The document outlines metabolic pathways and explains how enzymes function as catalysts to lower the activation energy of reactions. It also discusses factors that regulate enzyme activity such as substrate concentration, temperature, pH, and cellular mechanisms like phosphorylation.


![MetabolismMetabolism
Chapter 8
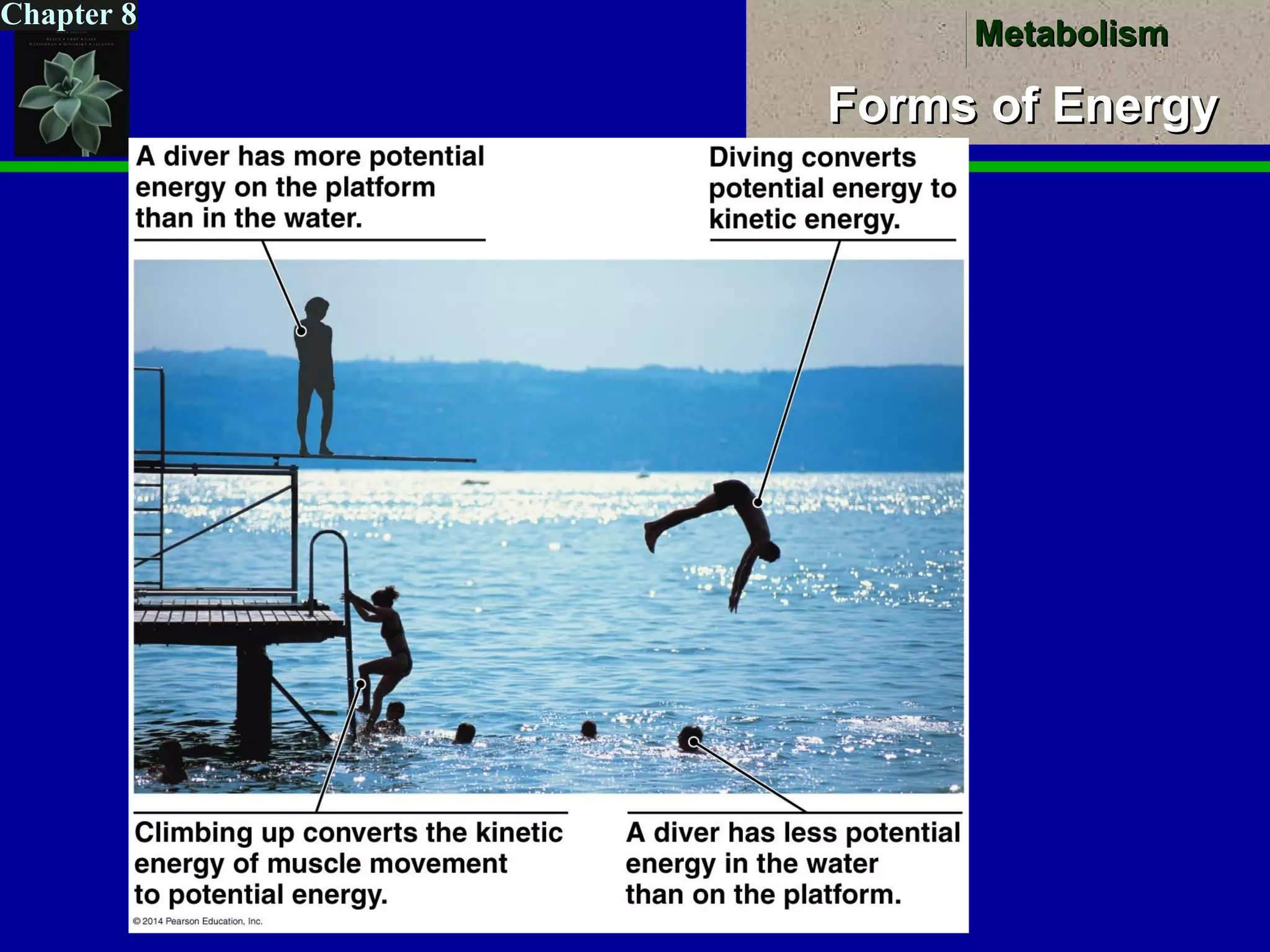
A. Two Forms of EnergyA. Two Forms of Energy
a. Kinetic Energya. Kinetic Energy = Energy of motion= Energy of motion
Examples: ?Examples: ?
b. Potentialb. Potential EnergyEnergy = Stored Energy= Stored Energy(can create motion(can create motion))
Examples: ?Examples: ?
- falling water, rolling bycicle ………..
- water behind a dam, stretch elastic, drawn bow….ect
** Important (biology) type of potential energy = Chemical E.
Examples: oil, gasoline = fossil fuels
(notes: write a chemical equation that depicts combustion
of a hydrocarbon [C16H32SO4]
Know these two definitions.](https://image.slidesharecdn.com/2ys17t4qp2z8ajjllzwc-signature-e326df53b4b03ba518749e799cd47c6c938c549643473522d3fab5605f310c89-poli-170301045819/75/Chapter-08-An-Introduction-to-Metabolism-6-2048.jpg)