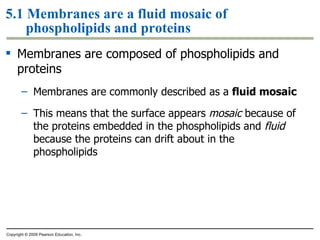
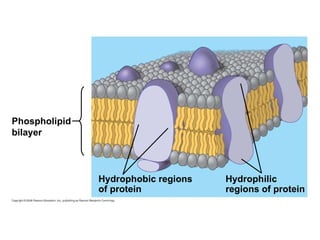
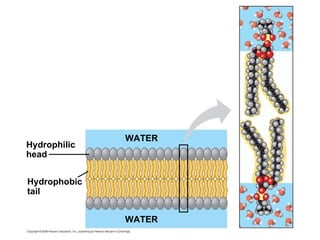
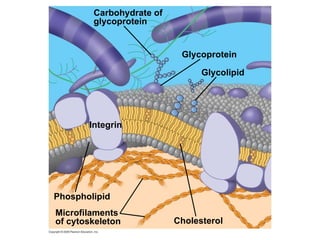
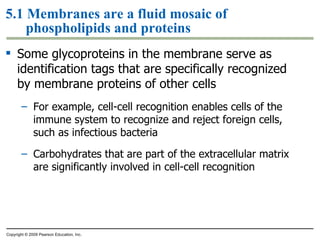
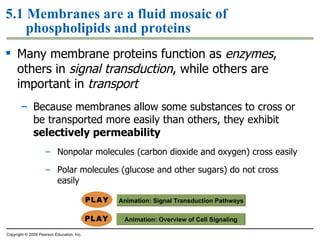
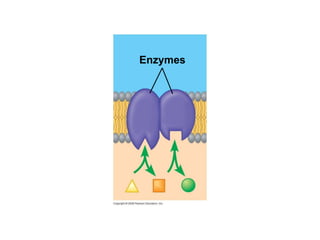
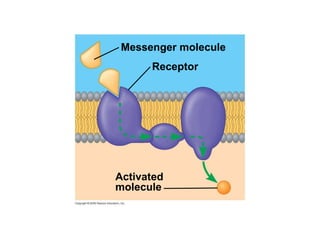
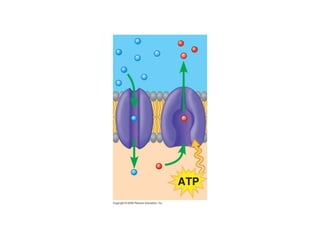
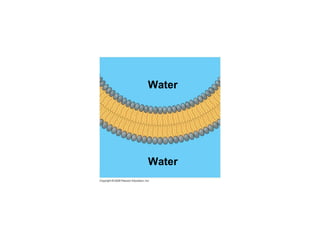
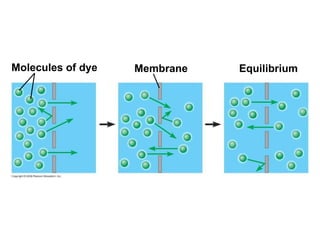
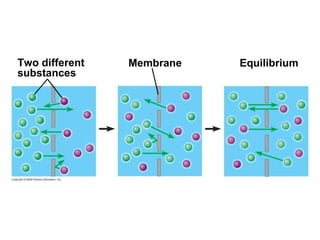
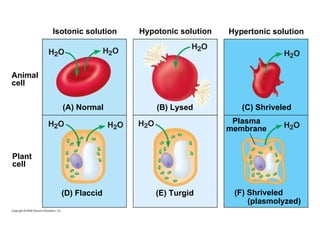
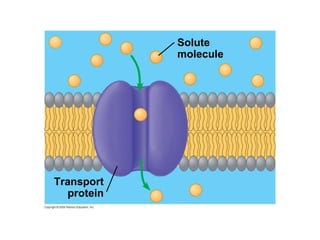
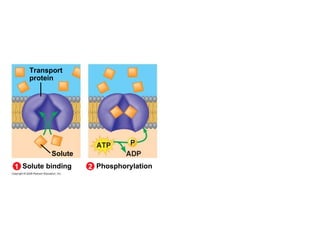
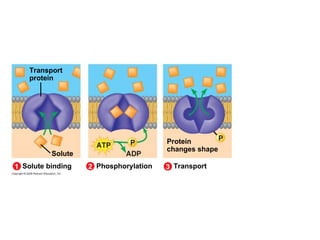
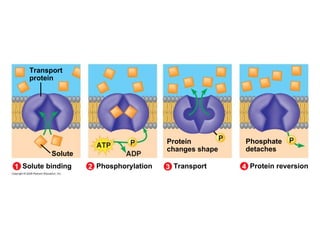
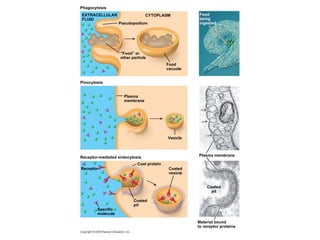
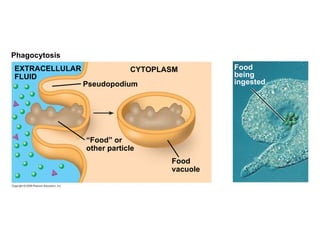
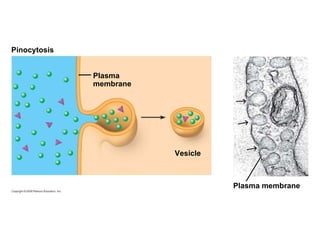
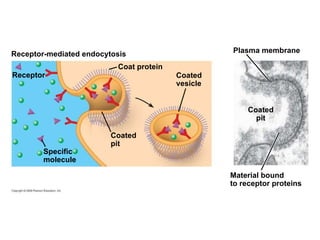
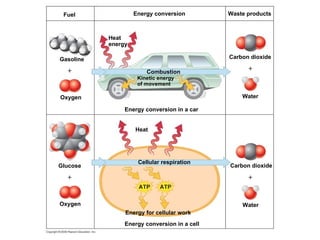
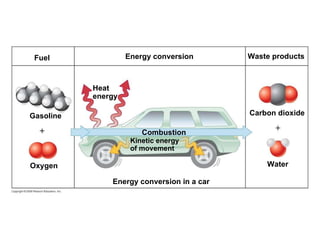
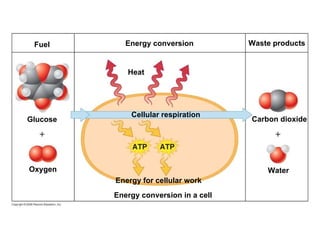
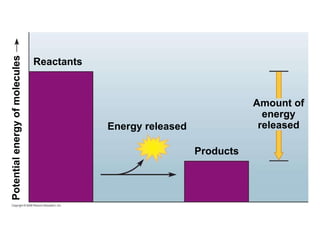
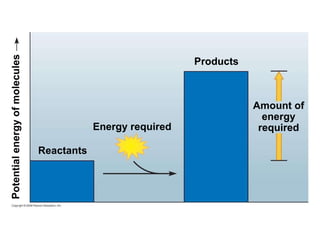
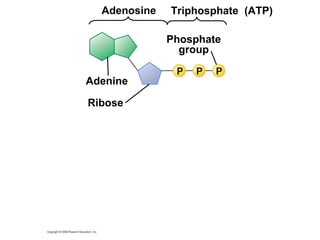
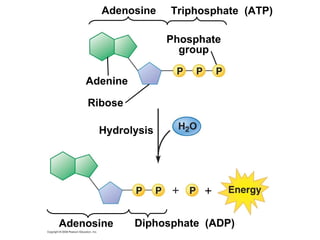
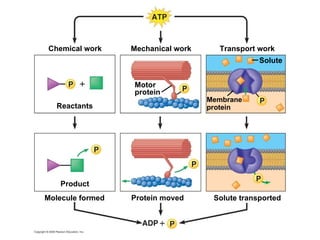
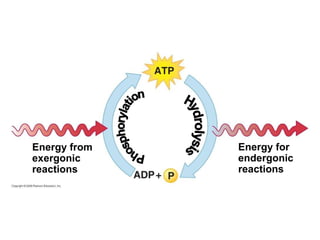
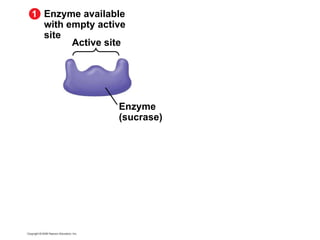
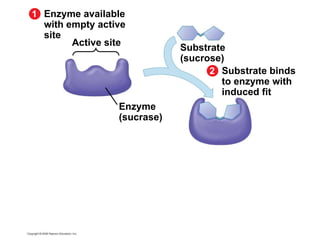
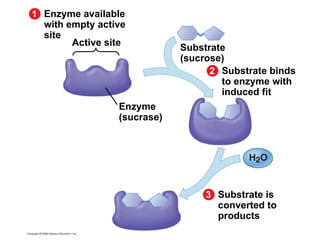
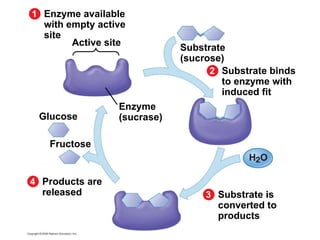
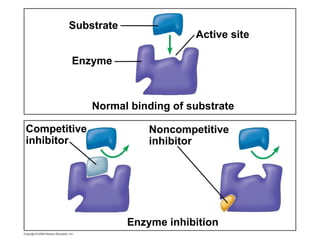
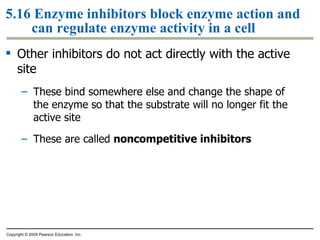
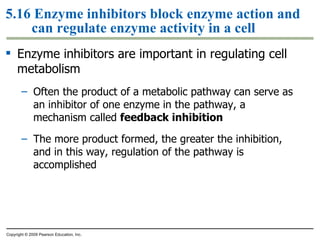
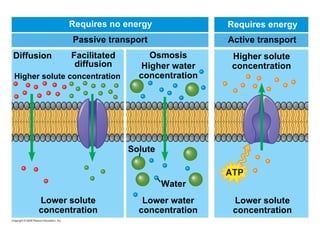
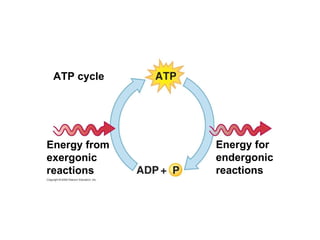
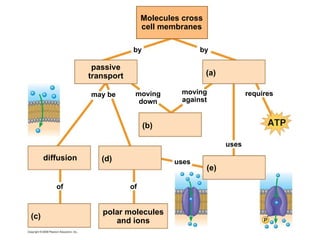
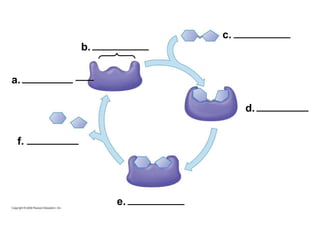
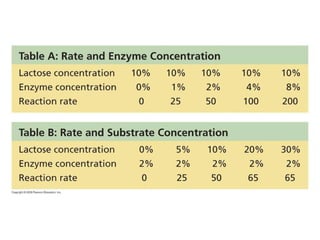
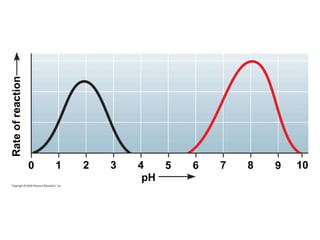
The document discusses cell membranes and their role in cellular processes. It covers how membranes are composed of phospholipids and proteins arranged in a fluid mosaic. Membranes allow selective permeability through diffusion, facilitated transport, and active transport. Transport proteins and vesicles move molecules across membranes. The chapter also addresses how cells use ATP and enzymes to drive energetic cellular processes and chemical reactions.