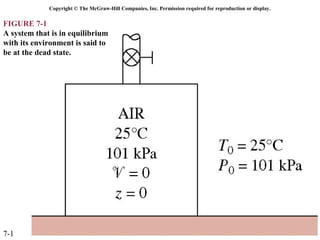
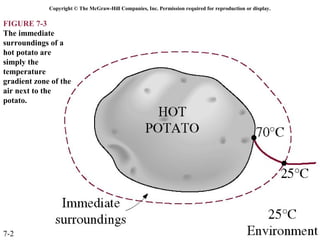
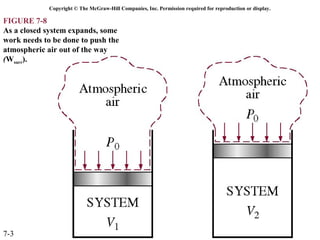
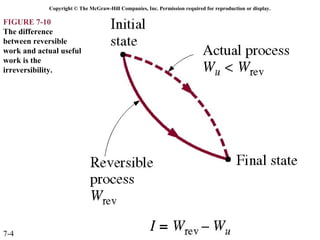
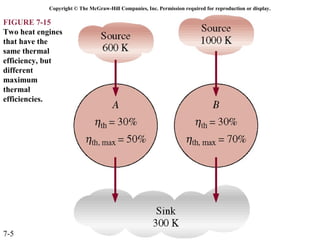
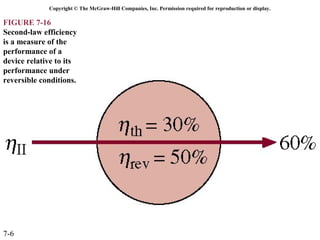
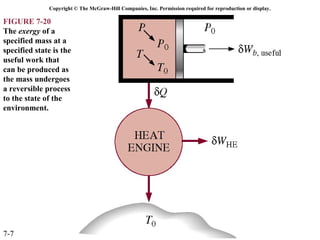
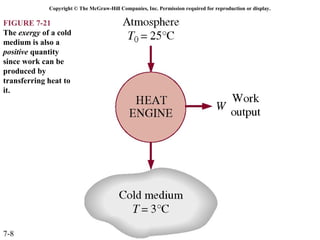
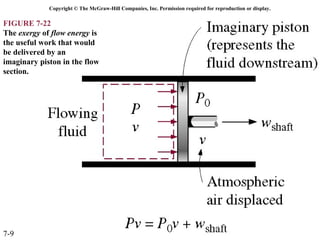
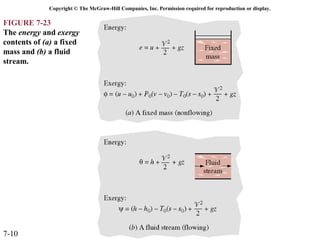
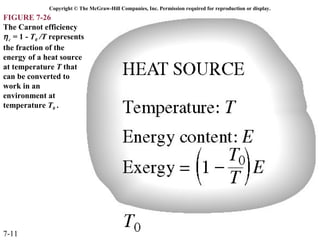
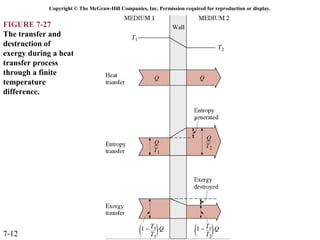
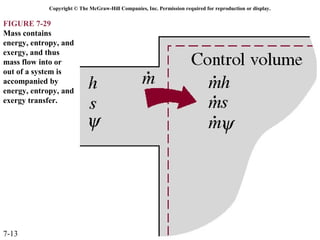
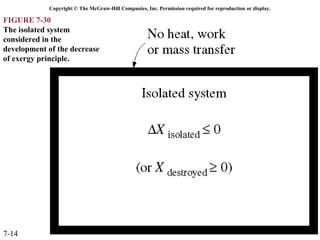
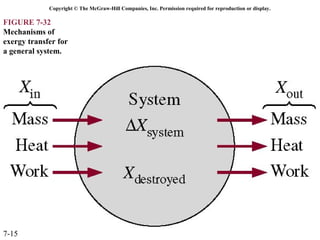
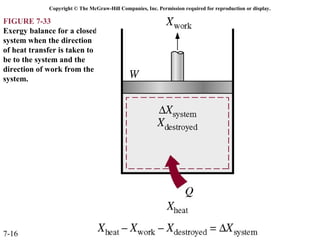
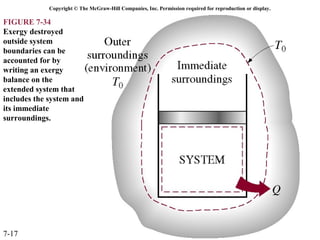
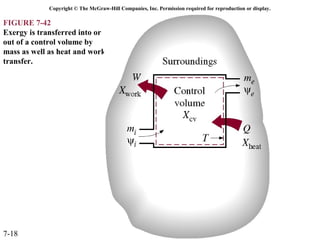
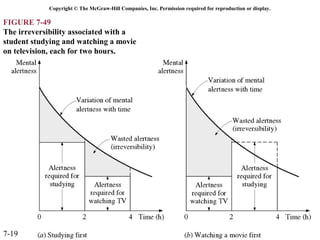
This document contains 20 figures from Chapter 7 describing concepts related to exergy, a measure of work potential. Exergy is defined as the useful work possible during a reversible process bringing a system to equilibrium with its environment. The figures address topics like the dead state, work potential of temperature gradients, reversible versus irreversible work, exergy transfers through heat, work and mass flows, and exergy balances for systems. Irreversibility and exergy destruction are also discussed.