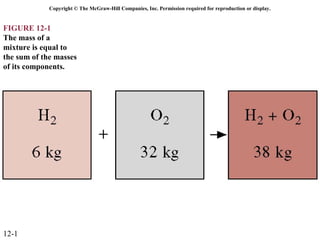
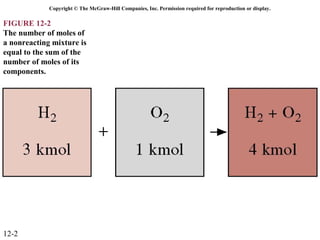
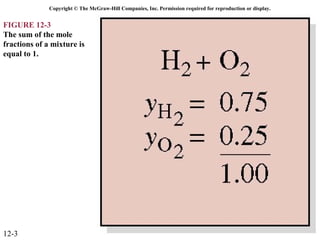
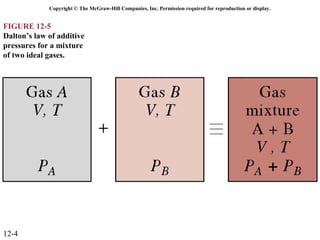
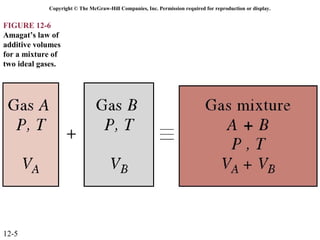
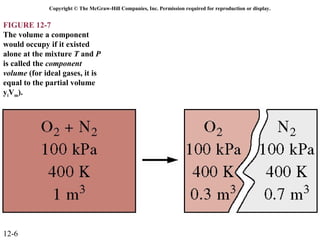
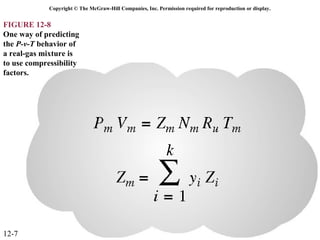
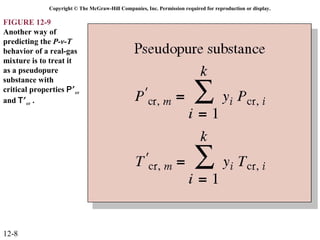
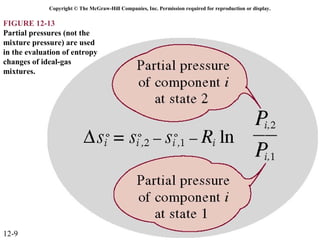
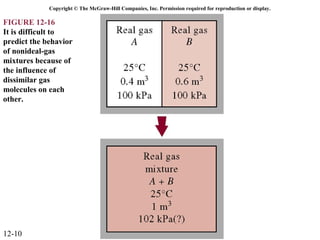
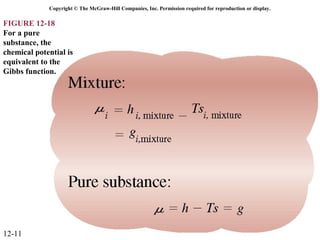
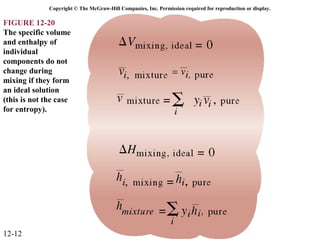
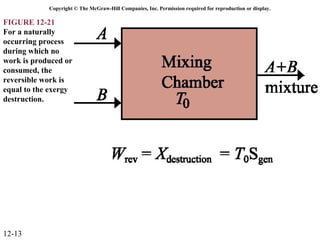
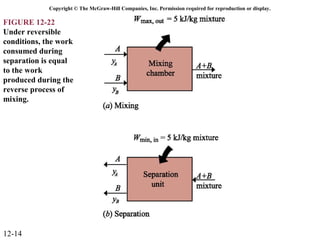
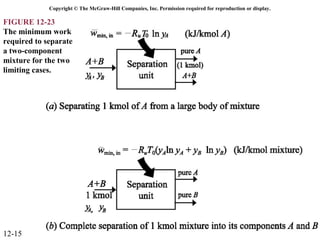
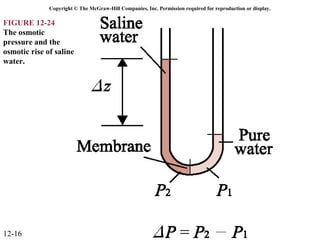
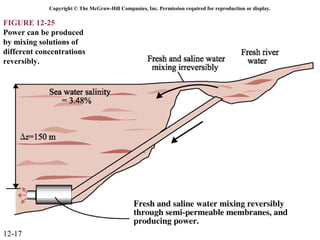
The document is a chapter about gas mixtures that includes 17 figures. It discusses various properties and behaviors of gas mixtures, including the mass, moles, mole fractions, pressures, volumes, entropy, and energy of mixtures. It also examines ideal and nonideal gas mixtures, the chemical potential of pure substances and mixtures, and mixing and separating processes related to mixtures.