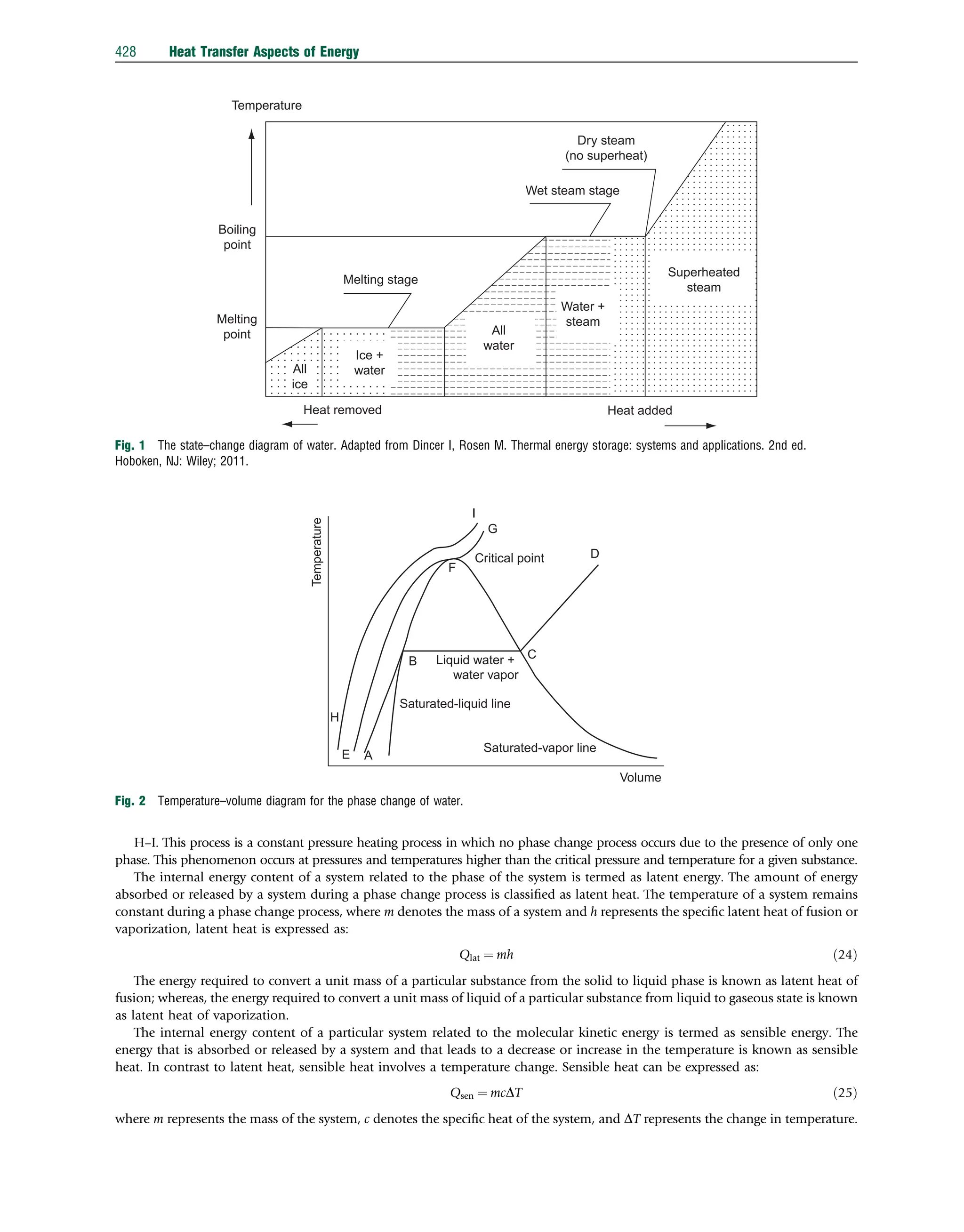
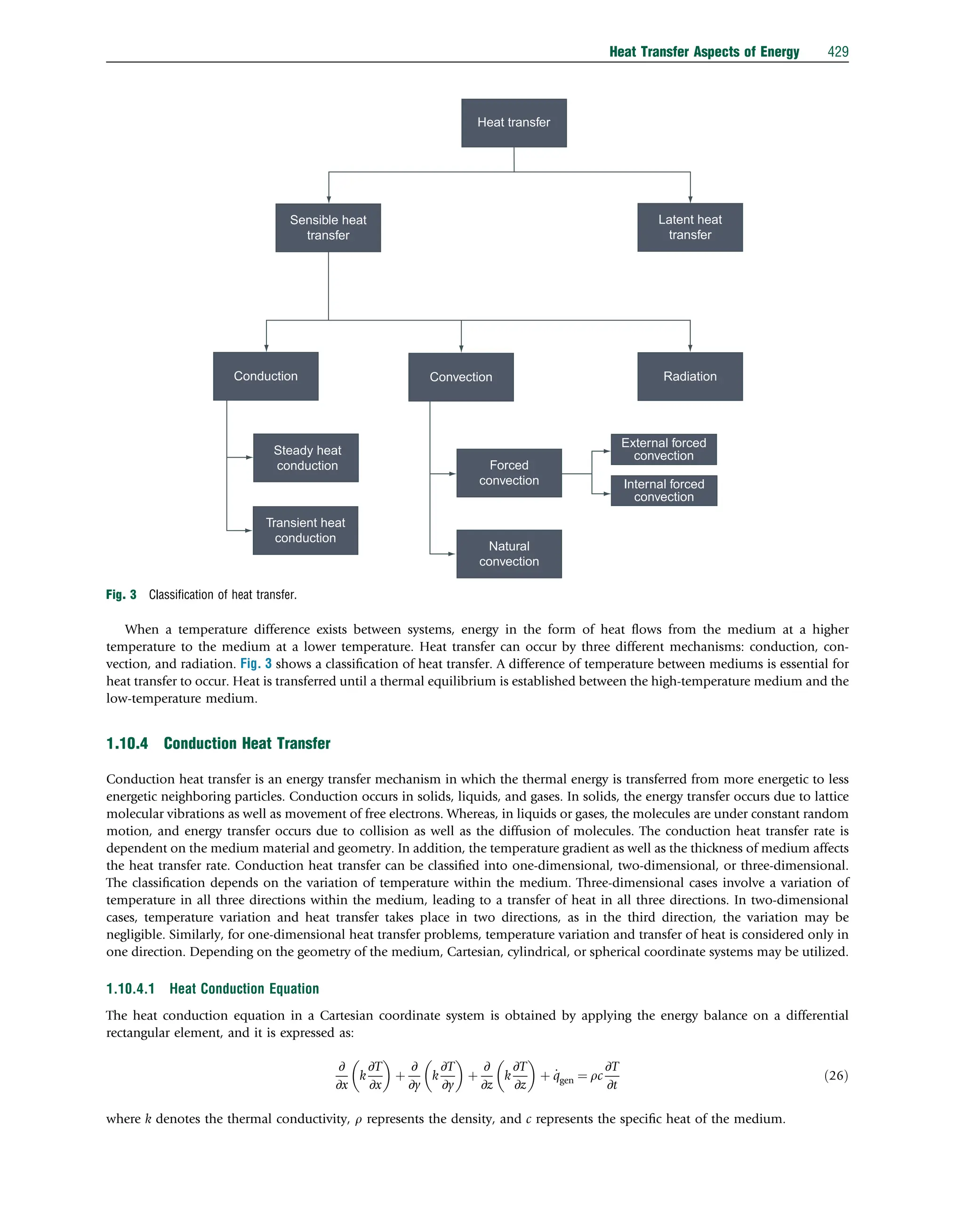
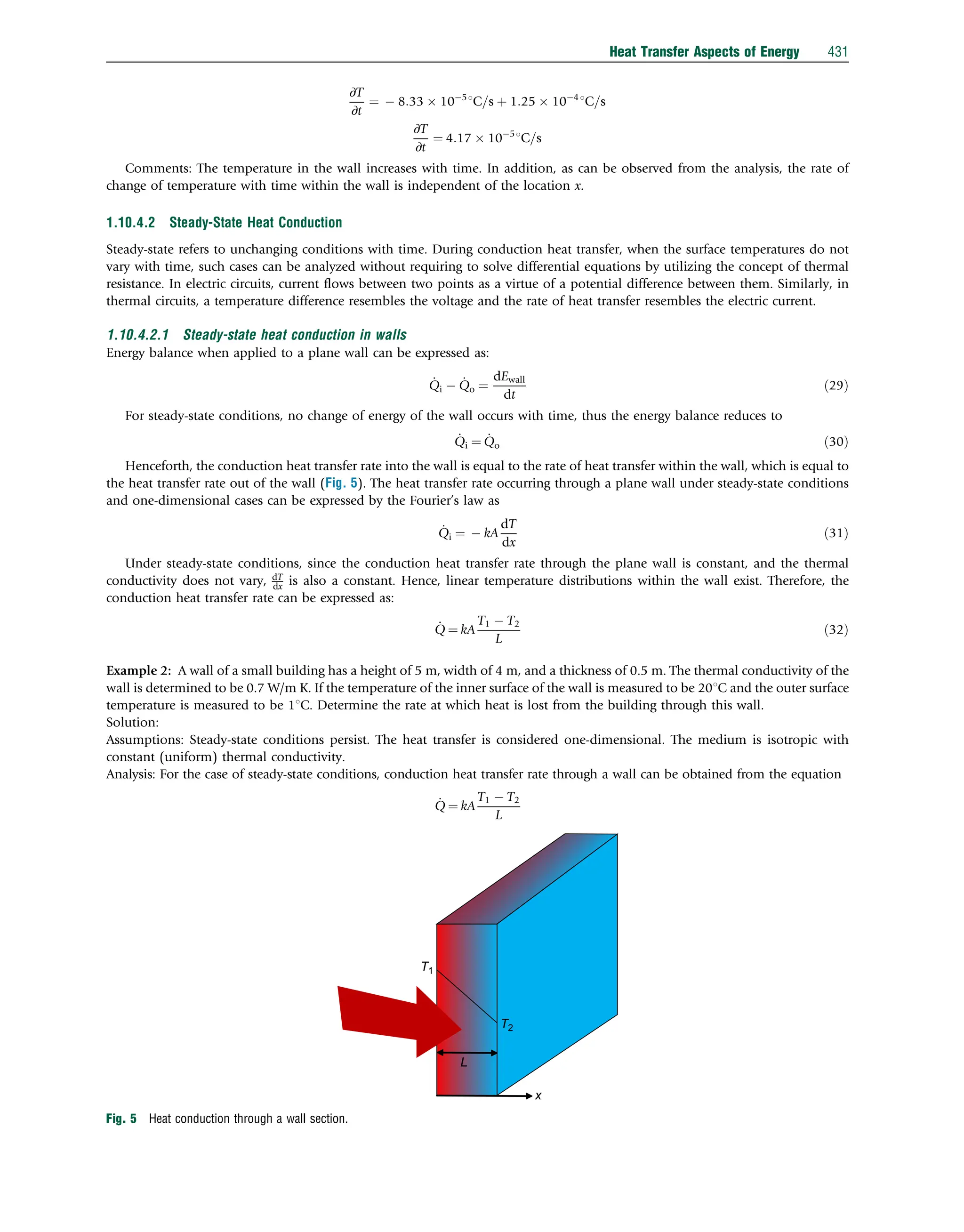
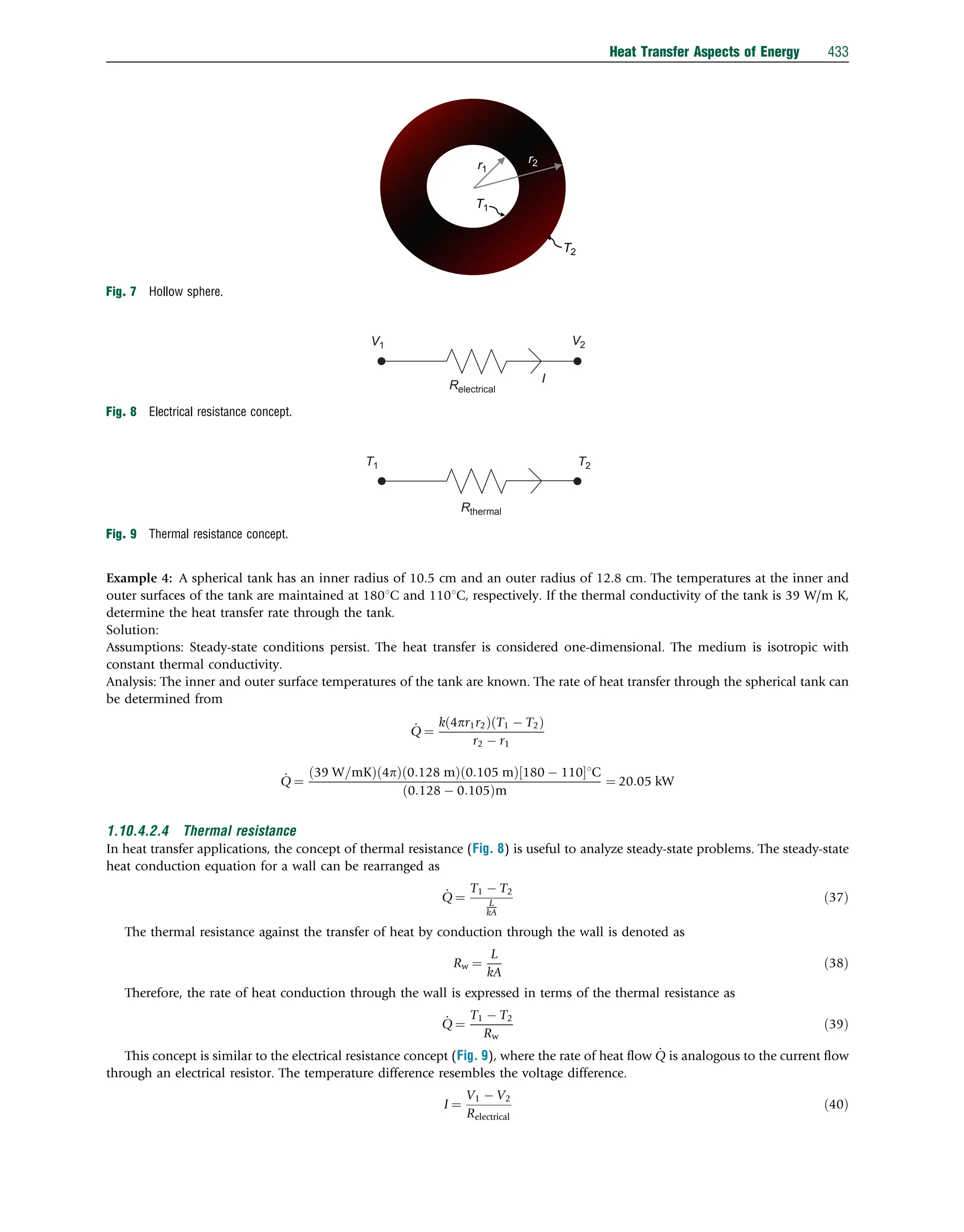
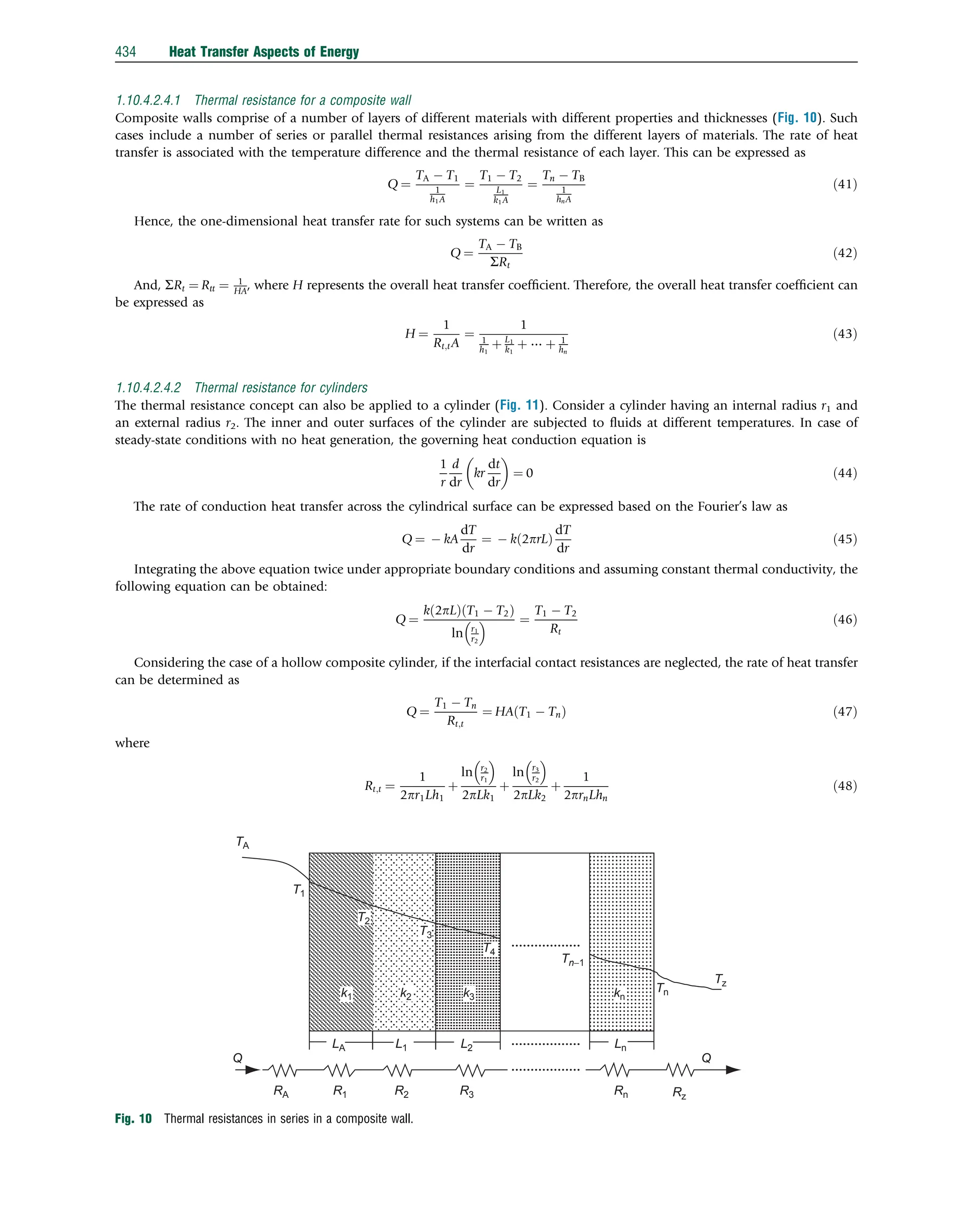
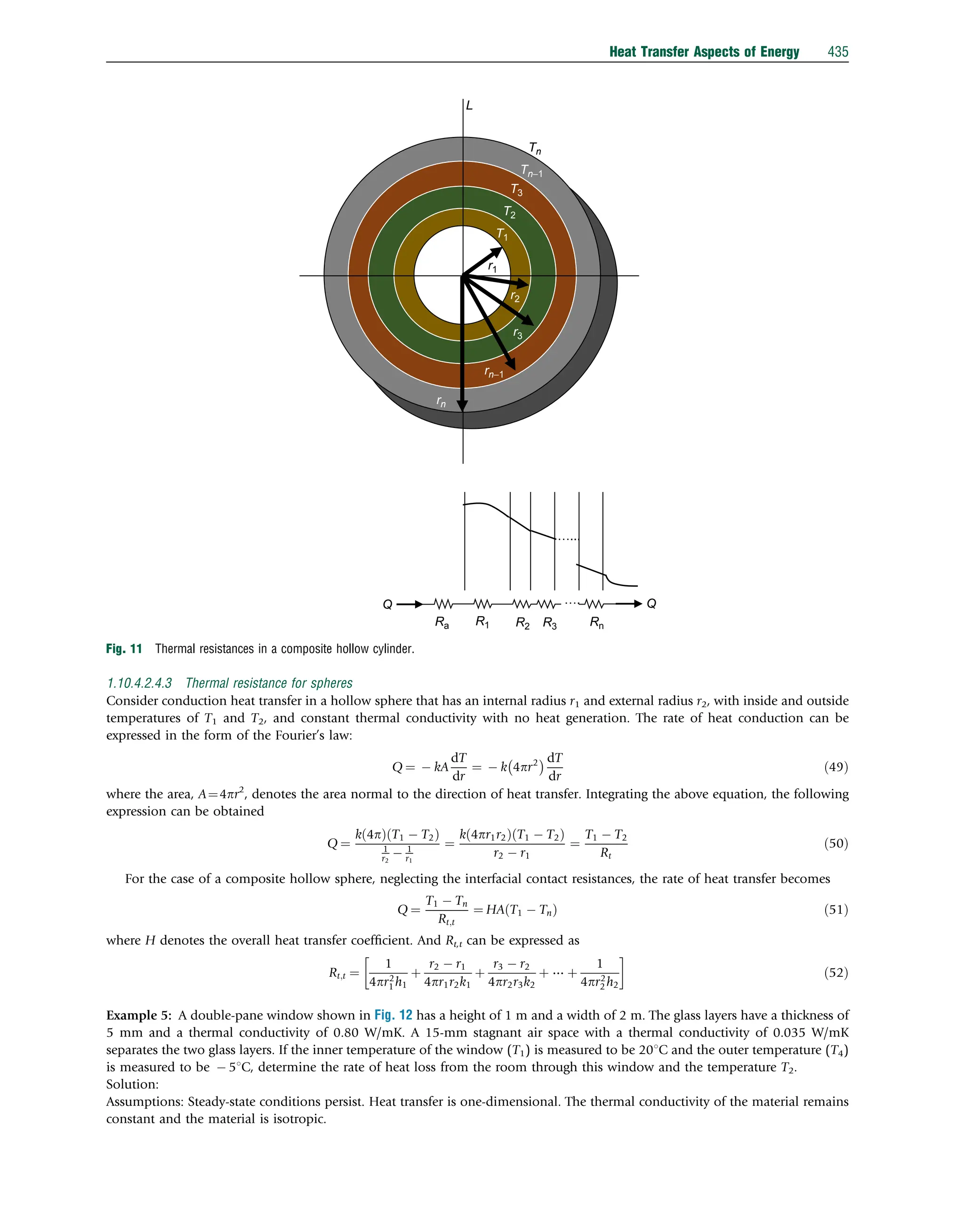
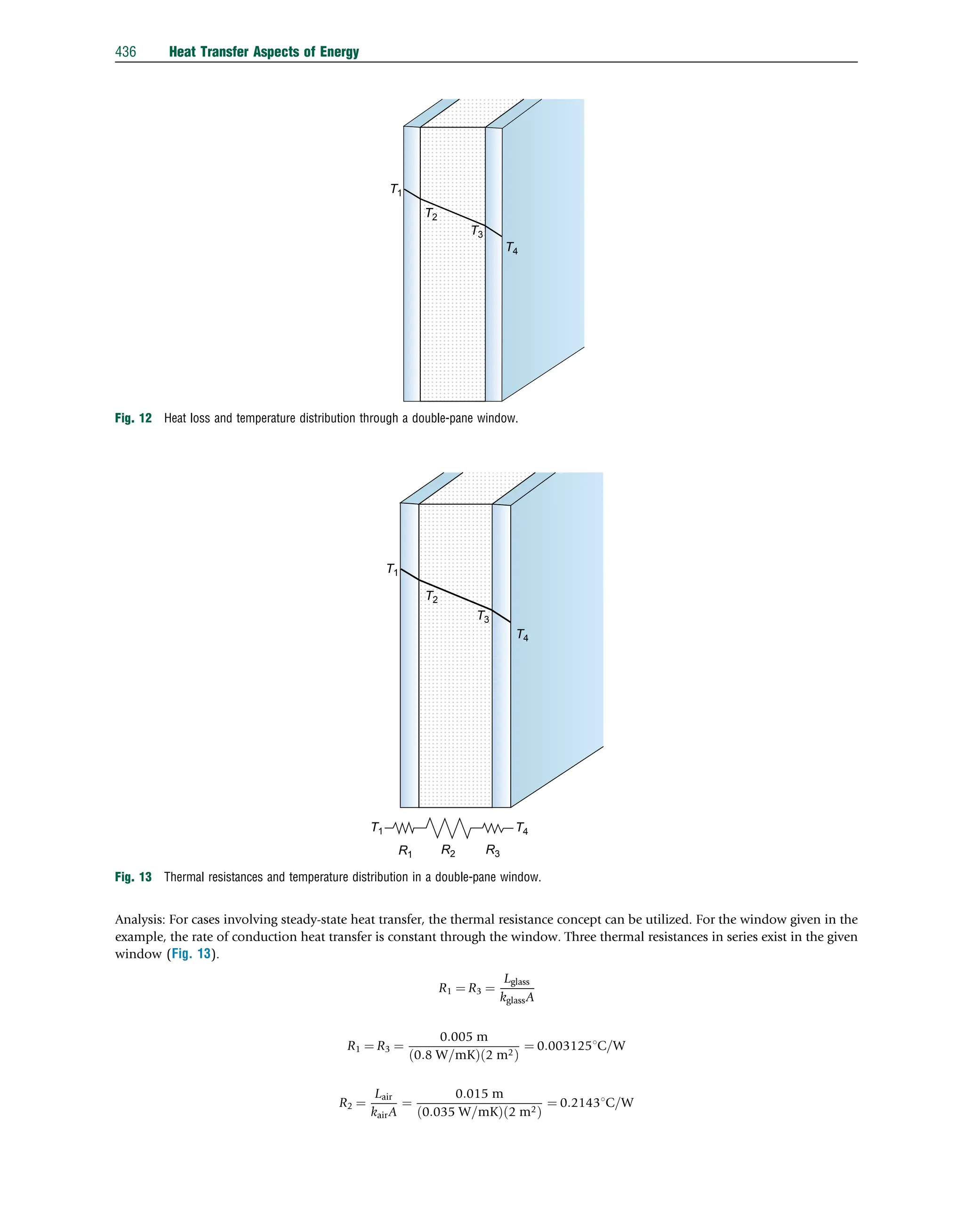
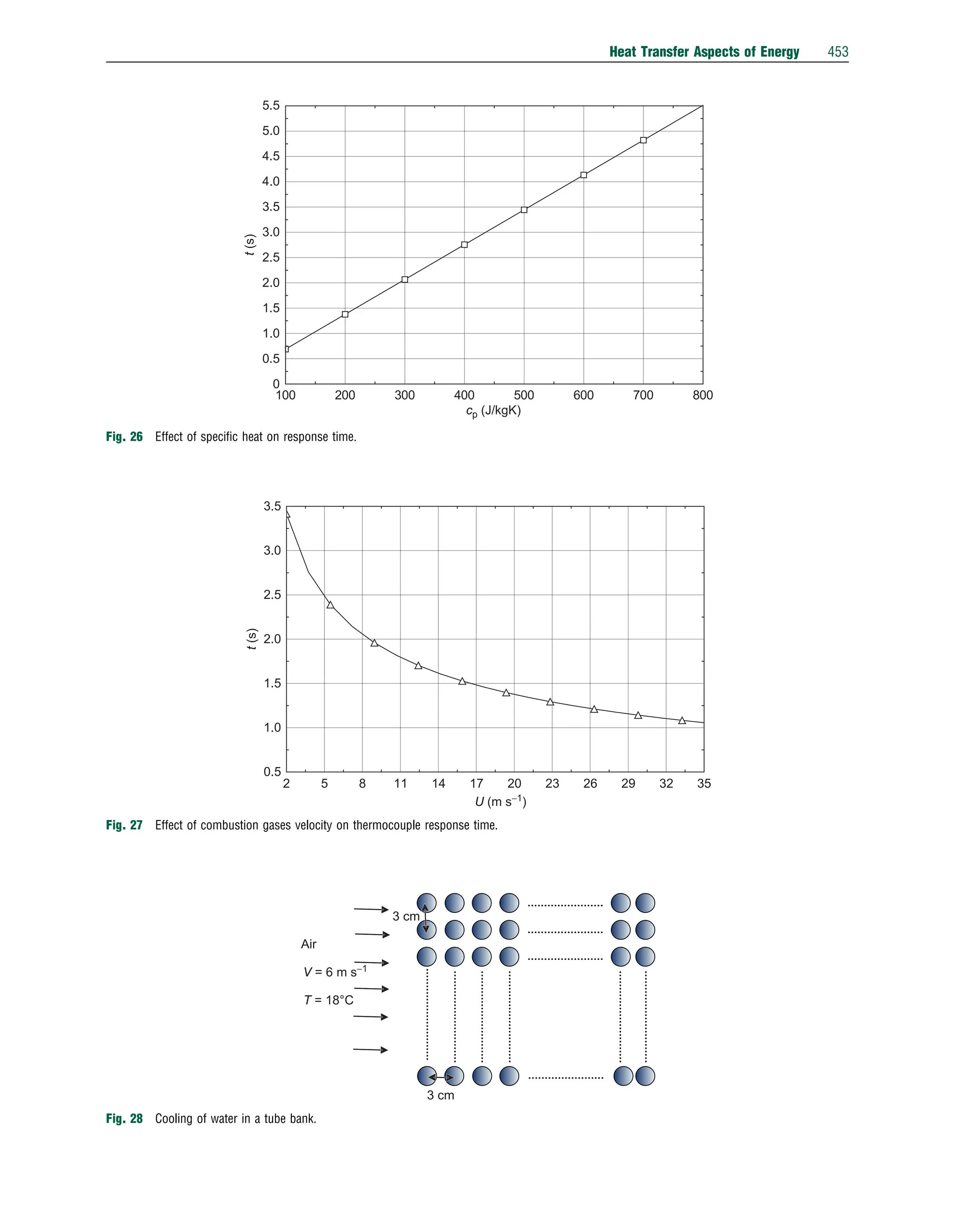
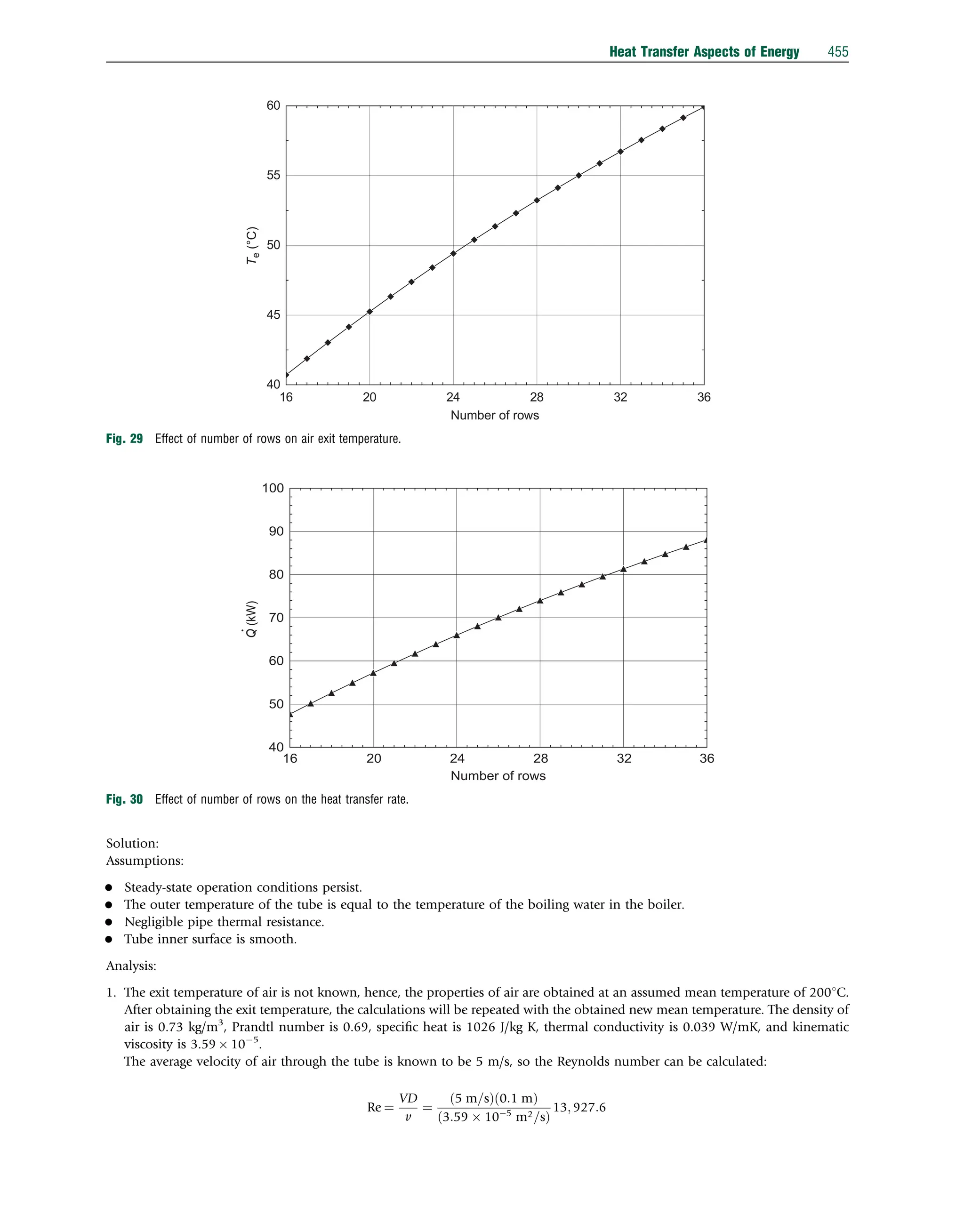
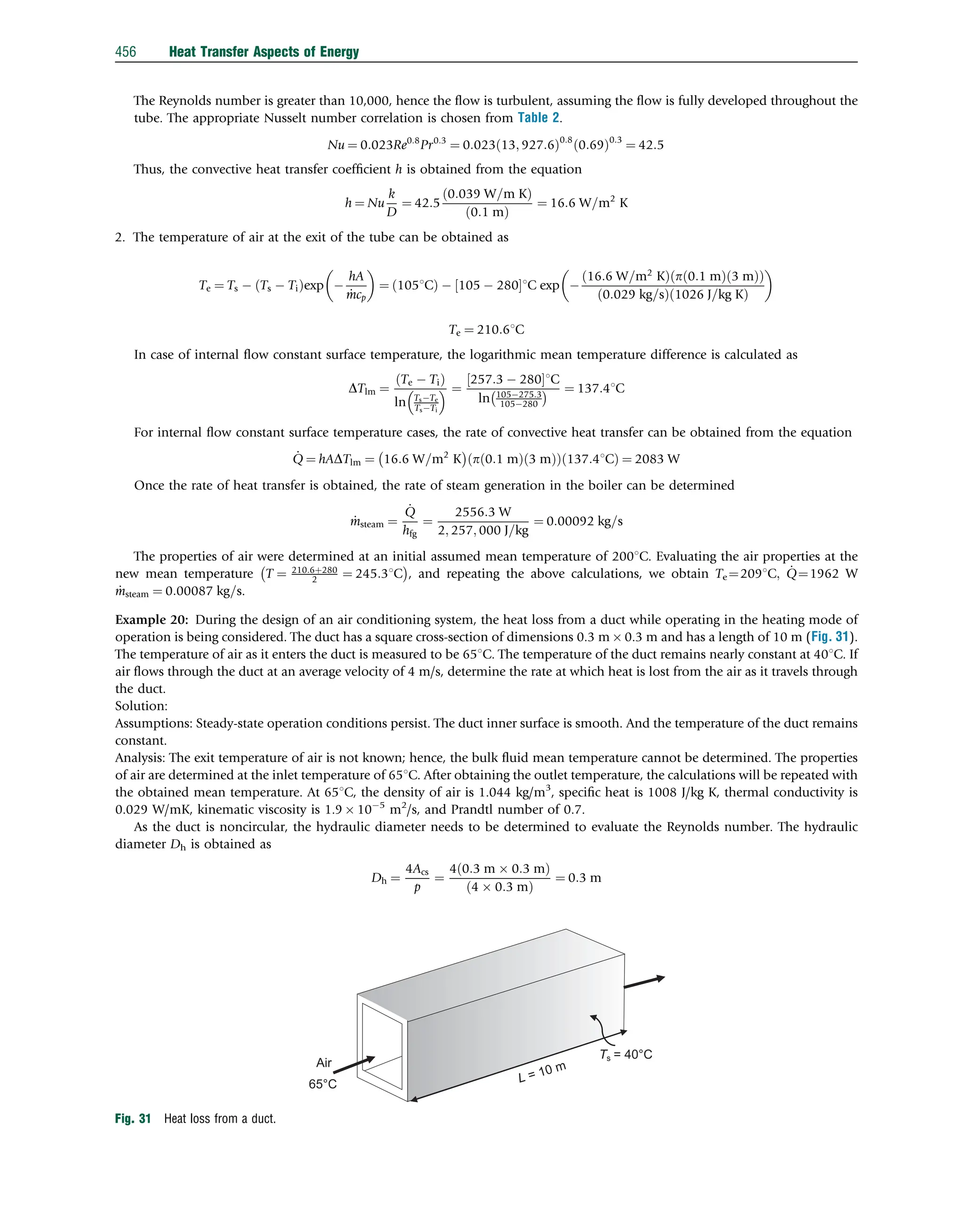
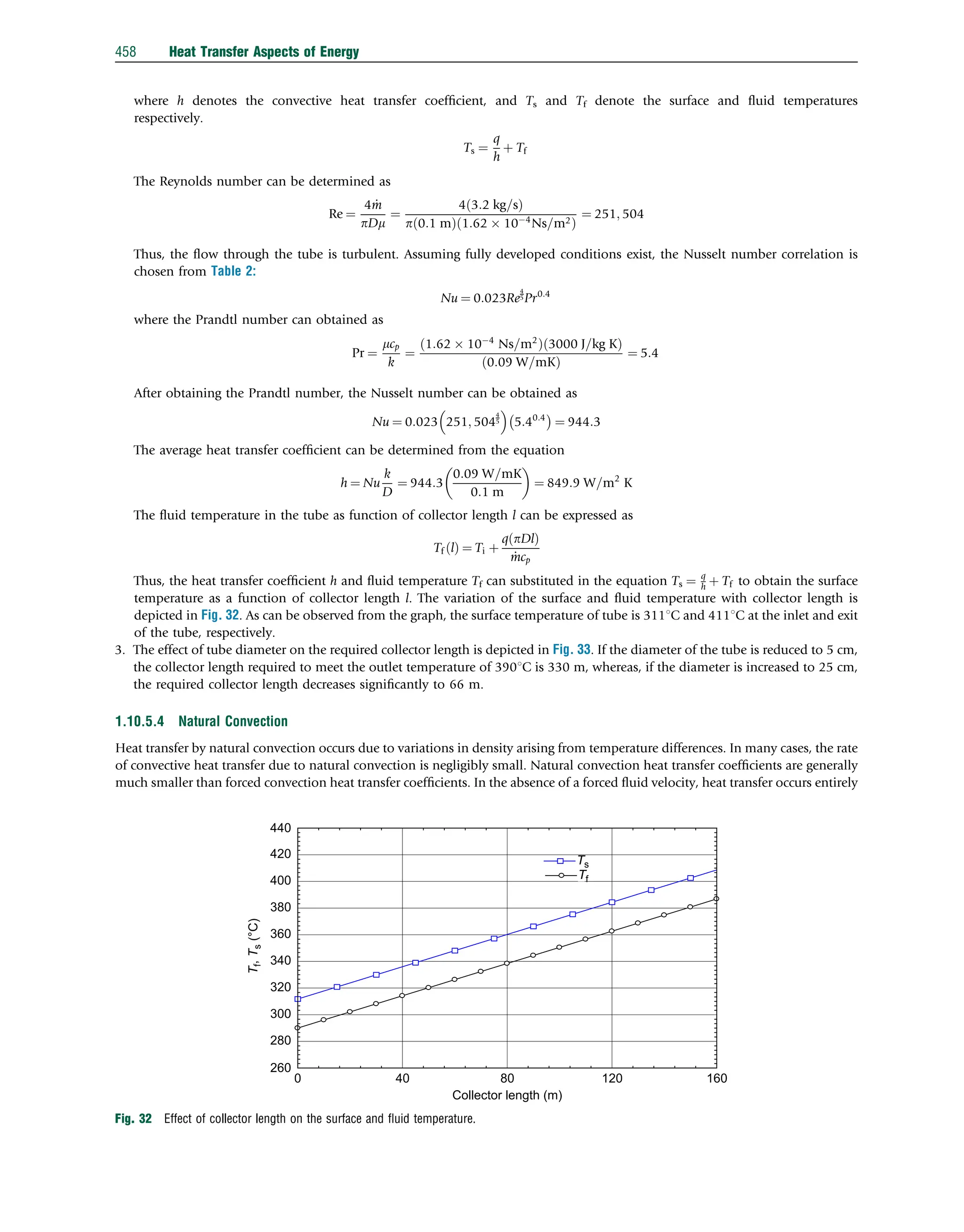
This document discusses heat transfer aspects of energy. It begins by introducing heat transfer and the underlying thermodynamic principles. The first law of thermodynamics states that energy cannot be created or destroyed, only changed from one form to another. Heat transfer occurs between bodies or systems at different temperatures until thermal equilibrium is reached. Various heat transfer mechanisms such as conduction, convection, and radiation are then examined. Case studies on heat exchangers and solar collectors are also presented to demonstrate applications of heat transfer concepts.








![Example 7: An individual dried fig was formed by hand as an infinite slab and refrigerated until the center temperature of 21.61C
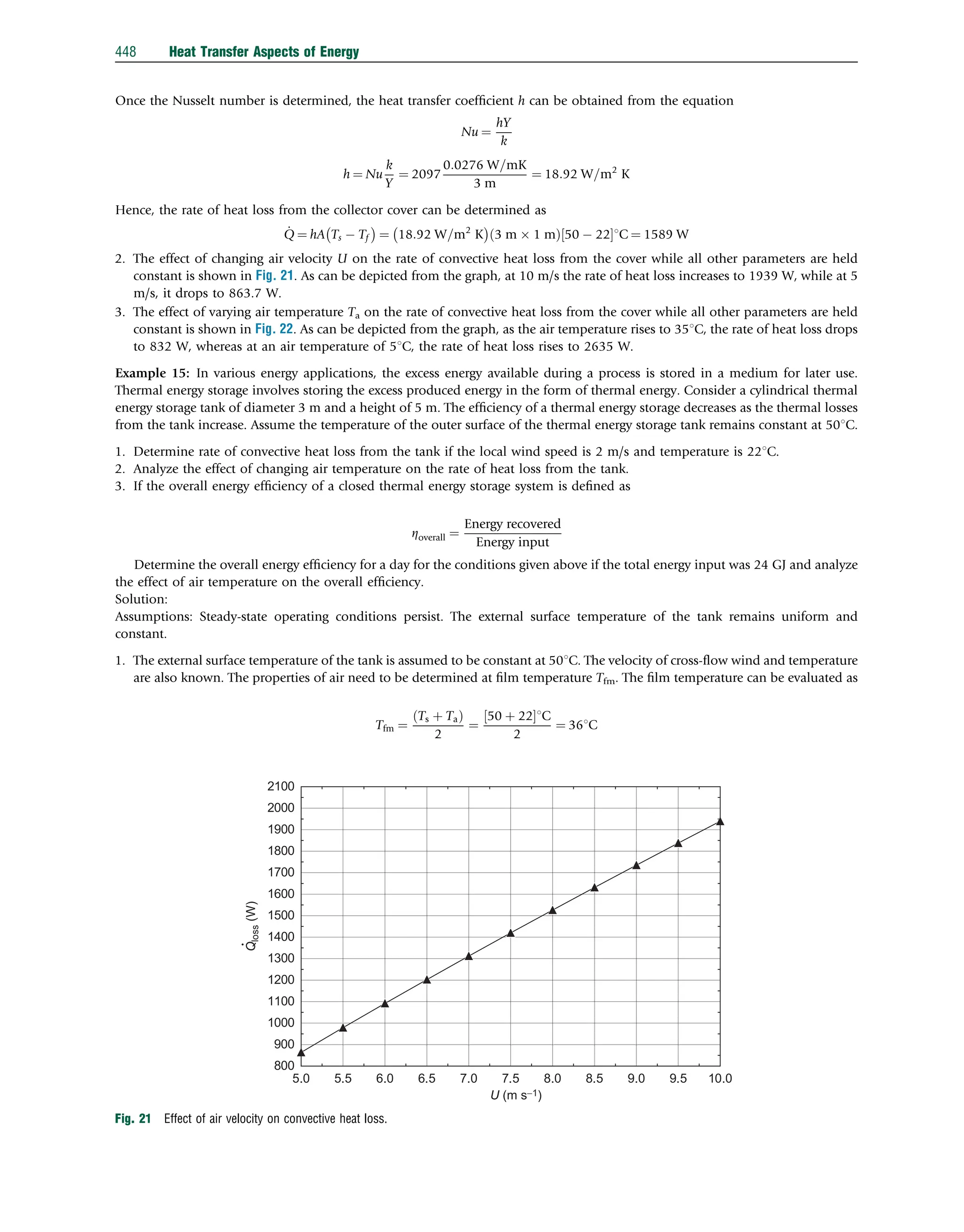
reached 221C in a freezer cabinet at Ta ¼ 221C. During this refrigeration process, the center temperature distribution of the
sample was measured at 30-s intervals, and the measurement was repeated three times. Some physical and thermal properties
are L¼0.01 m, l¼0.05 m, X¼0.04 m, k¼0.2367 W/m 1C, a¼9.88 108
m2
/s, and h¼9.045 W/m2
1C. Further information on
the experiments and analysis technique, along with the relevant data can be found in Ref. [1]. Here, we will compute the center
temperature distribution of this slab product and compare it with the experimental data.
Solution: From the data we have, the Biot number is calculated as Bi ¼ hl
k ¼ 9:045 0:005=0:2367 ¼ 0:191 here, the Biot number
is between 0.1rBir100, leading to a convection boundary condition problem. By knowing the Biot number, the values of m1 and
A1 can be found as m1 ¼0.422 and A1 ¼1.03. The theoretical dimensionless center temperature is calculated from the following
equation, considering Fo40.2:
yt ¼ A1B1 ¼ 2Bi Bi2
þ m2
1
0:5
=m1 Bi2
þ Bi þ m2
1
h i
exp m2
1Fo
¼ 1:03 exp 0:4222
Fo
where Fo¼at/l2
¼9.88 108
t/0.0052
.
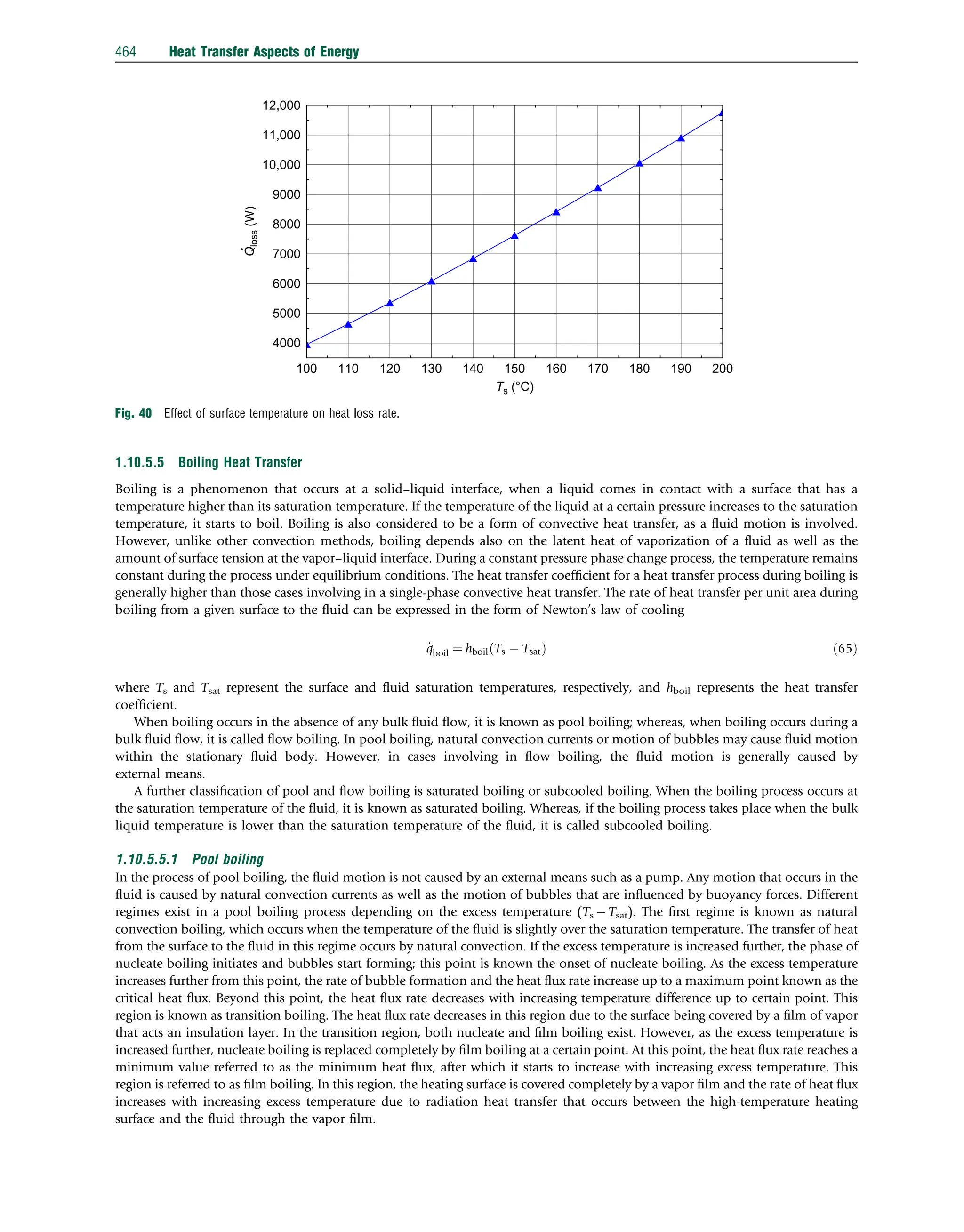
The results are shown in Fig. 14.
Example 8: An individual cylindrical eggplant was cooled until the center temperature of 221C reached 71C in the water pool of a
hydro cooling unit at a temperature of 11C. During the cooling process, the center temperature distribution of the product was
measured at 30-s intervals. Some physical and thermal properties of the product are: R¼0.0225 m, J¼0.142 m, k¼0.6064 W/m
1C, a¼1.438 107
m2
/s, and h¼44.15 W/m2
1C. Further information on the experiments and analysis technique, along with the
relevant data, can be found in Ref. [2]. Here, we will determine the theoretical center temperature distribution of the cylindrical
eggplant and compare it with the experimental data.
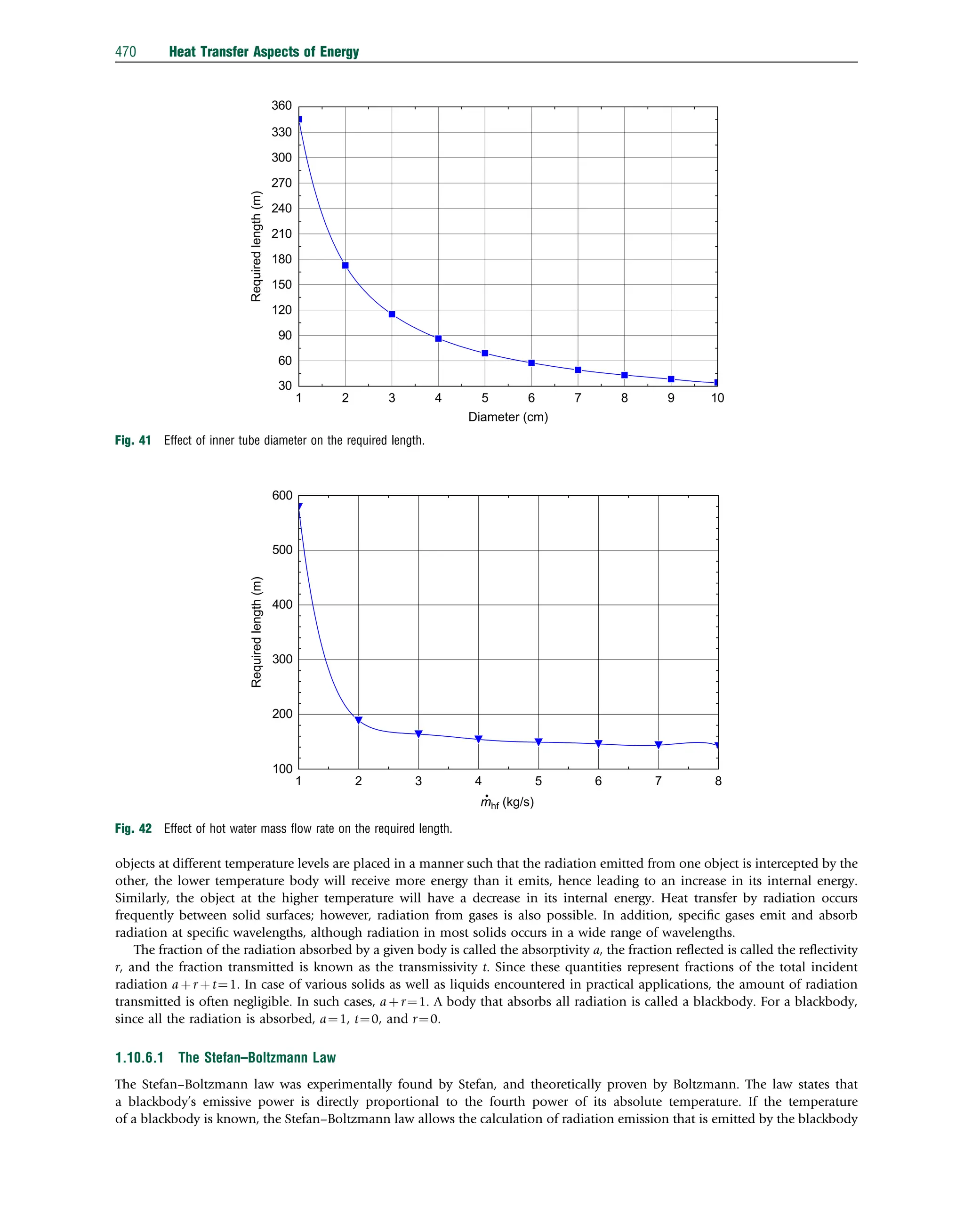
Table 1 Dimensionless transient temperatures
Equations or series solutions
For Bio0.1
y¼eBiFo
For 0.1rBir100
Infinite slab
y ¼
P
1
n ¼ 1
AnBnCn
An ¼ 1
ð Þnþ1
2Bi Bi2
þ m2
n
0:5
=mn Bi2
þ Bi þ m2
n
Bn ¼ exp m2
nFo
Cn ¼ cosmnG
Infinite cylinder
y ¼
P
1
n ¼ 1
AnBnCn
An ¼ 2Bi= m2
n þ Bi2
J0 mnR
ð Þ
Bn ¼ exp m2
nFo
Cn ¼ J0 mnG
ð Þ
Sphere
y ¼
P
1
n ¼ 1
AnBnCn An ¼ 1
ð Þnþ1
2Bi m2
n þ Bi 1
ð Þ2
h i0:5
= Bi2
Bi þ m2
n
Bn ¼ exp m2
nFo
Cn ¼ sinmnG=mnG
For Bi4100
Infinite slab
y ¼
P
1
n ¼ 1
AnBnCn
An ¼ 1
ð Þnþ1
2=mn
ð Þ
Bn ¼ exp m2
nFo
Cn ¼ cosmnG
mn ¼ 2n 1
ð Þp=2
Infinite cylinder
y ¼
P
1
n ¼ 1
AnBnCn
An ¼ 2=mnJ1 mn
ð Þ
Bn ¼ exp m2
nFo
Cn ¼ J0 mnG
ð Þ
J0 mnG
ð Þ ¼ 1 m2
n=22
þ m4
n=22
42
m6
n=22
42
62
Sphere
y ¼
P
1
n ¼ 1
AnBnCn
An ¼ 2 1
ð Þnþ1
Bn ¼ exp m2
nFo
Cn ¼ sinmnG=mnG
mn ¼ np
438 Heat Transfer Aspects of Energy](https://image.slidesharecdn.com/13comprehensiveenergysystems-240412072703-d37af1a8/75/Comprehensive-energy-systems-pdf-Comprehensive-energy-systems-pdf-17-2048.jpg)
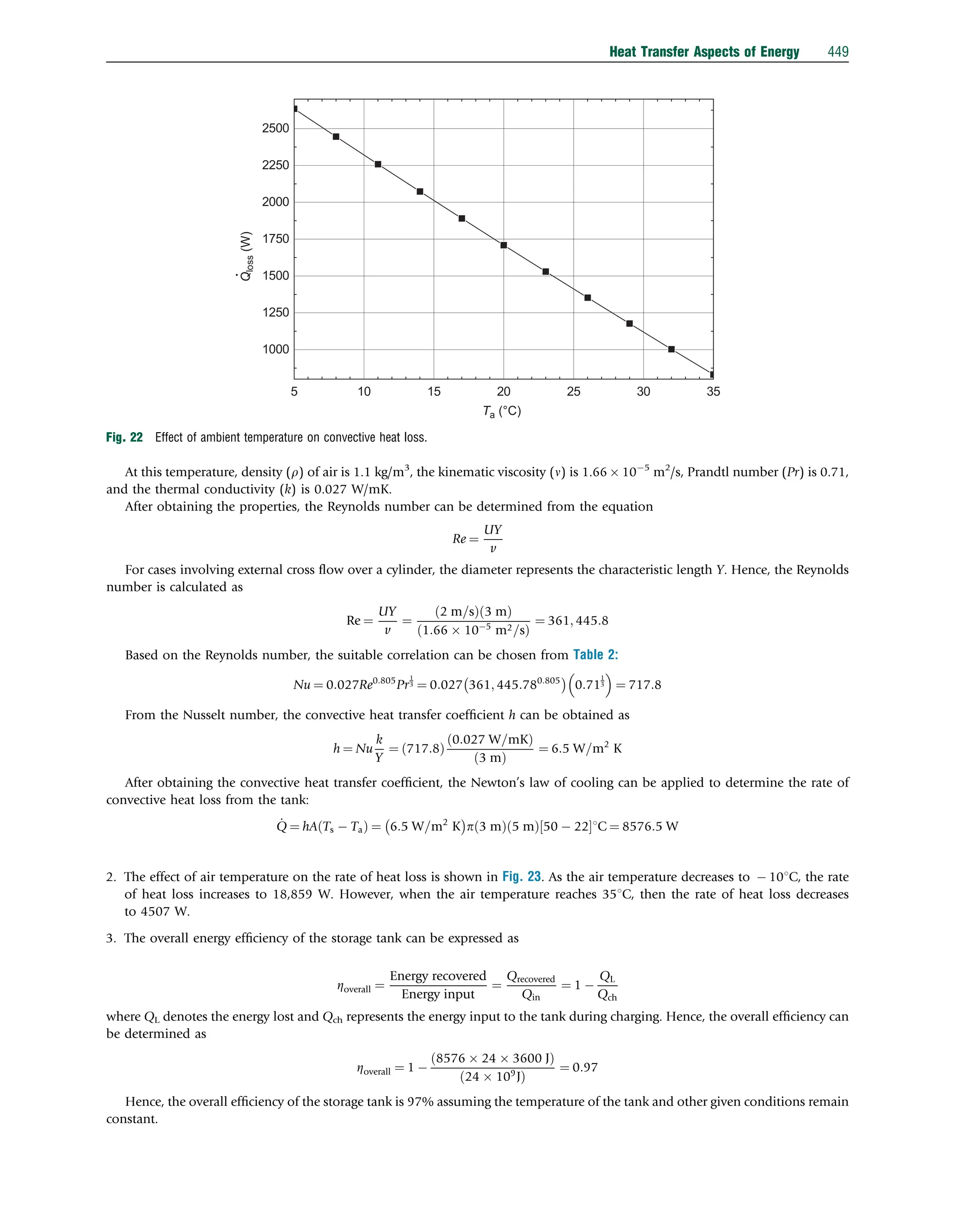
![Here, the Biot number is between 0.1rBir100, leading to a convection boundary condition problem. By knowing the Biot
number as 1.64, the values of mn and An for n¼1 6, which are considered sufficient terms, are taken as m1¼1.497, m2 ¼4.219,
m3 ¼7.242, m4 ¼10.332, m5 ¼13.445 and m6 ¼16.571 and A1 ¼1.297, A2 ¼ 0.427, A3¼0.203, A4 ¼ 0.119, A5 ¼0.0823, and
A6 ¼ 0.0589. The theoretical dimensionless center temperature is calculated from the following equation, considering Fo40.2:
yt ¼
X
1
n ¼ 1
AnBn ¼
X
6
n ¼ 1
Anexp m2
nFo
¼ 1:297 exp 1:4972
Fo
0:427 exp 4:2192
Fo
þ 0:203 exp 7:2422
Fo
0:119 exp 10:3322
Fo
þ 0:0823 exp 13:4452
Fo
0:0589 exp 16:5712
Fo
where Fo¼at/R2
¼1.438 107
t/0.02252
.
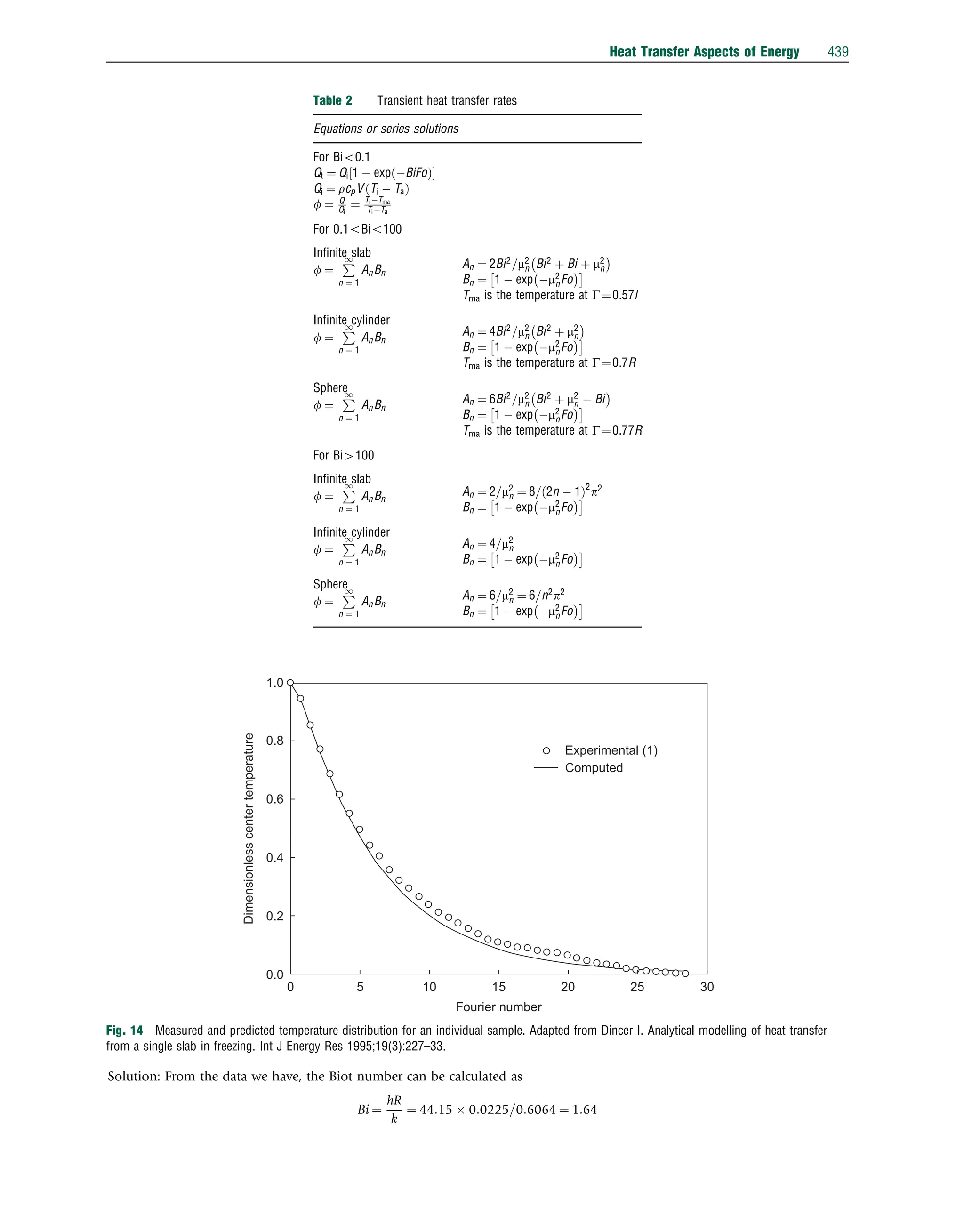
The computed results are shown in Fig. 15. The experimental results can also be obtained from Ref. [2].
Example 9: An individual spherical pear was cooled until the center temperature of 221C reached 21C in the water pool of a hydro
cooling unit at a temperature of 11C. During the cooling process, the center temperature distribution of the product was measured
at 30-s intervals. Some physical and thermal properties of the product are: R¼0.03 m, k¼0.5527 W/m 1C, a¼1.378 107
m2
/s,
and h¼160.56 W/m2
1C. Further information on the experiments, and analysis technique, along with the relevant data, can be
found in Ref. [2]. Here, we will determine the theoretical center temperature distribution of the spherical pear and compare it with
the experimental data.
Solution: From the data we have, the Biot number can be calculated as
Bi ¼
hR
k
¼ 160:56 0:030=0:5572 ¼ 8:64
Here, the Biot number is between 0.1rBir100, leading to a convection boundary condition problem. By knowing the Biot
number as 8.64, the values of mn and An for n¼1–6, which are considered sufficient terms, are taken as m1 ¼2.79, m2 ¼5.646,
m3 ¼8.581, m4 ¼11.578, m5 ¼14.618 and m6 ¼17.618 and A1 ¼1.903, A2 ¼ 1.657, A3¼ 1.42, A4 ¼ 1.196, A5 ¼1.018, and
A6 ¼ 0.878. The theoretical dimensionless center temperature is calculated from the following equation, considering Fo40.2:
yt ¼
X
1
n ¼ 1
AnBn ¼
X
6
n ¼ 1
Anexp m2
nFo
¼ 1:903 exp 2:792
Fo
1:675 exp 5:6462
Fo
þ 1:42exp 8:5812
Fo
1:196 exp 11:5782
Fo
þ 1:018 exp 14:6182
Fo
0:878 exp 17:6862
Fo
where Fo¼at/R2
¼1.378 107
t/0.032
. The computed results are shown in Fig. 16, the experimental results can also be obtained
from Ref. [2].
Example 10: Steel billets are used extensively in the manufacturing of various products for numerous industries. The heat
treatment process of steel billets involves heating the billets to a temperature of 8001C and immersing them in a water bath until
they are cooled to a specified temperature. The billets have a diameter of 6.5 cm and a length of 100 cm. The thermal conductivity
1.0
0.8
0.6
0.4
Dimensionless
center
temperature
0.2
0.0 0.1 0.2
Fourier number
0.3 0.4 0.6
0.5 0.7
Fig. 15 Theoretical dimensionless temperature profiles for the center of an individual eggplant.
440 Heat Transfer Aspects of Energy](https://image.slidesharecdn.com/13comprehensiveenergysystems-240412072703-d37af1a8/75/Comprehensive-energy-systems-pdf-Comprehensive-energy-systems-pdf-19-2048.jpg)



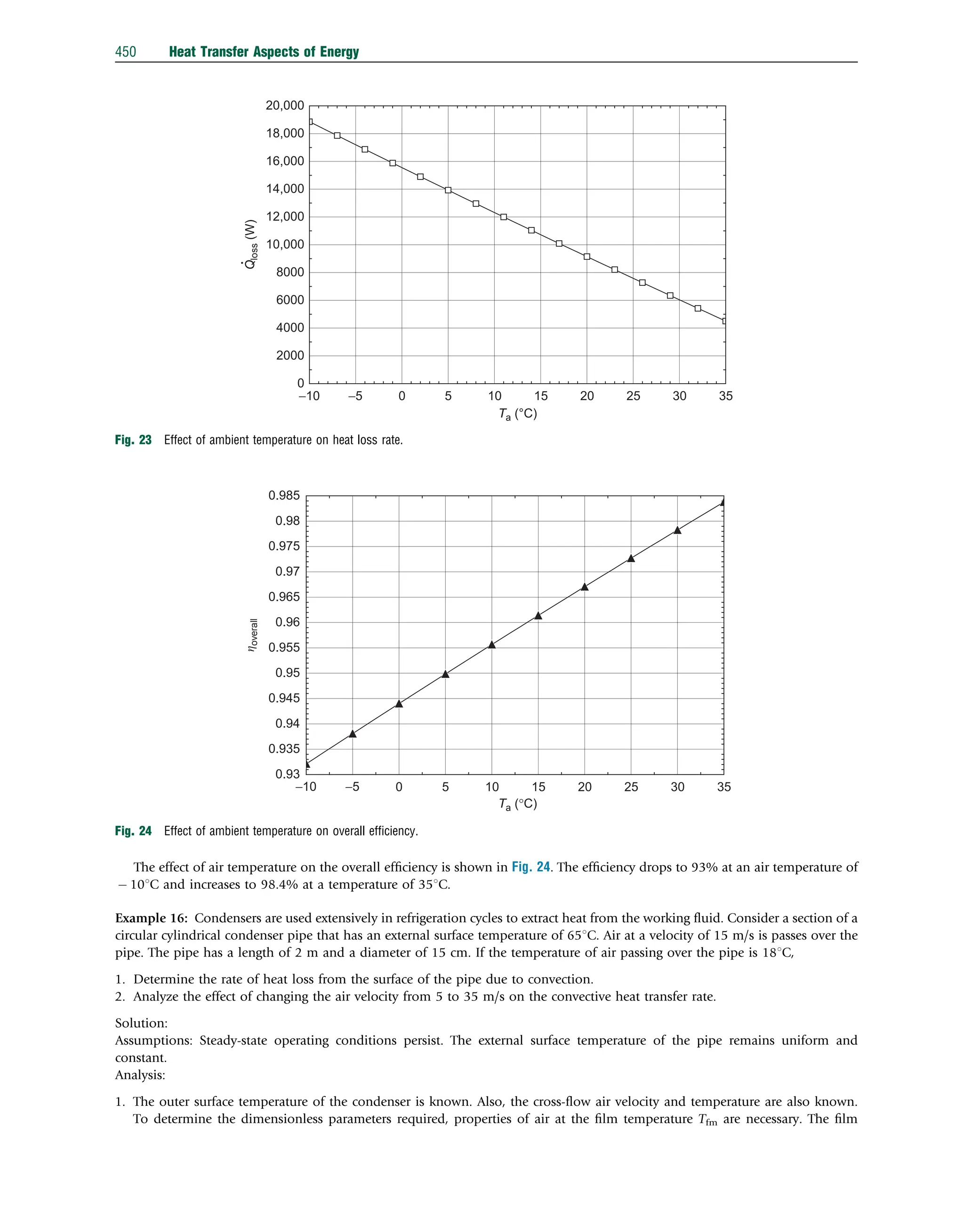
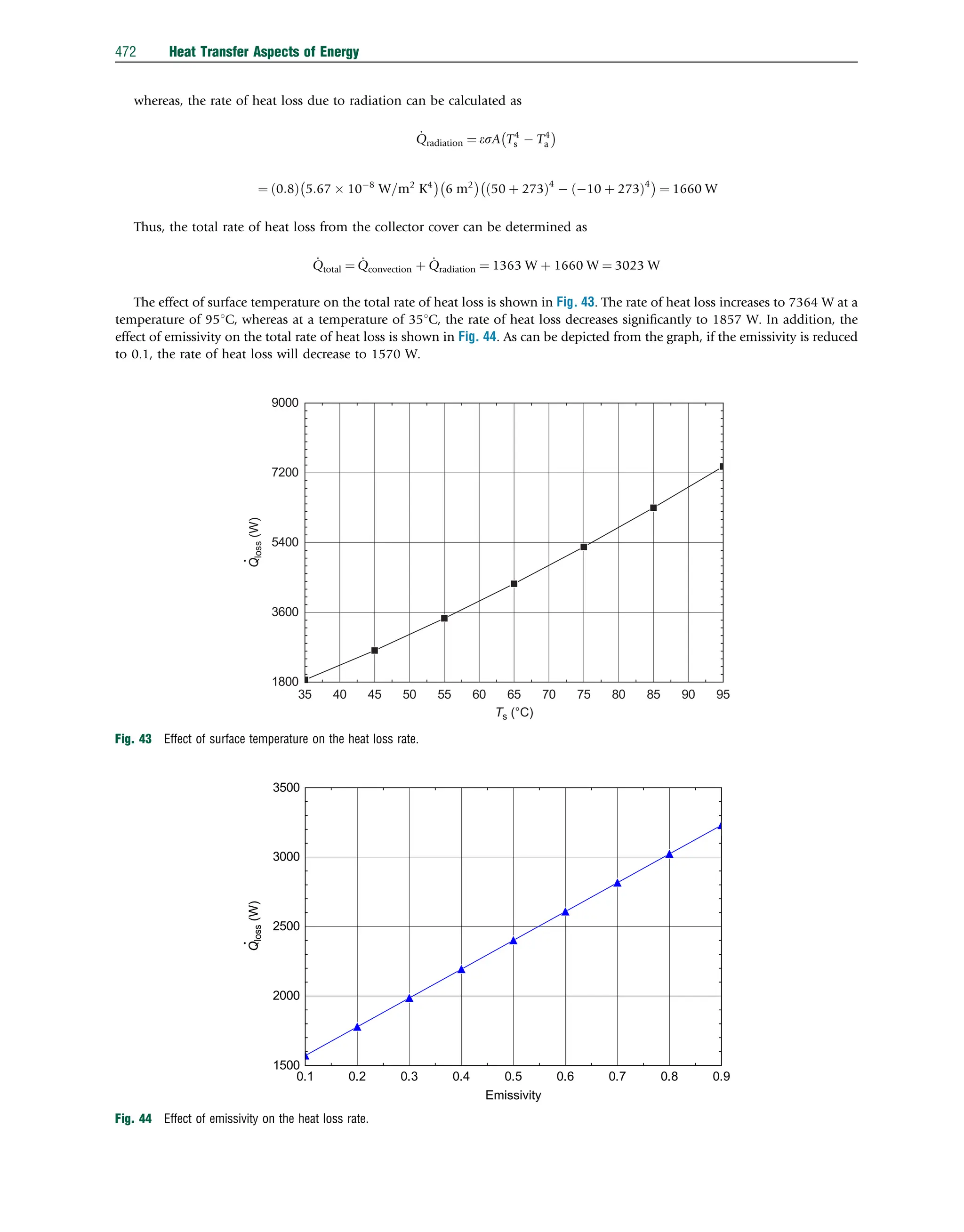
![The Reynolds number at the plate end can be determined as follows:
Re ¼
UY
v
¼
8:3 m=s
ð Þ 3m
ð Þ
ð1:66 105
Þm2=s
¼ 15 105
45 105
Since the Reynolds number is greater than the critical Reynolds number, a suitable correlation to be used for this case is
Nu ¼ 0:037Re
4
5 871
Pr
1
3 ¼ 0:037 15 105
4
5
871
0:71
1
3 ¼ 2097
Table 4 Forced convection heat transfer correlations and equations
Equation or correlation
Correlations for external flow over a flat plate
Nu¼0.332Re1/2
Pr1/3
for PrZ0.6 for laminar; local; Tfm
Nu¼0.664Re1/2
Pr1/3
for PrZ0.6 for laminar; average; Tfm
Nu¼0.565Re1/2
Pr1/2
for Prr0.05 for laminar; local; Tfm
Nu¼0.0296Re4/5
Pr1/3
for 0.6rPrr60 for turbulent; local; Tfm, Rer108
Nu¼(0.037Re4/5
871)Pr1/3
for 0.6oPro60 for mixed flow; average; Tfm, Rer108
Correlations for external cross-flow over circular cylinders
Nu¼cRen
Pr1/3
for PrZ0.7 for average; Tfm; 0.4oReo4 106
where
c¼0.989 and n¼0.330 for 0.4oReo4
c¼0.911 and n¼0.385 for 4oReo40
c¼0.683 and n¼0.466 for 40oReo4000
c¼0.193 and n¼0.618 for 4000oReo40,000
c¼0.027 and n¼0.805 for 40,000oReo400,000
Nu¼cRen
Prs
(Pra/Prs)1/4
for 0.7oPro500 for average; Ta; 1oReo106
where
c¼0.750 and n¼0.4 for 1oReo40
c¼0.510 and n¼0.5 for 40oReo1000
c¼0.260 and n¼0.6 for 103
oReo2 105
c¼0.076 and n¼0.7 for 2 105
oReo106
s¼0.37 for Prr10
s¼0.36 for Pr410
Nu¼0.3 þ [(0.62Re1/2
Pr1/3
)/(1 þ (0.4/Pr)2/3
)1/4
][1 þ (Re/28,200)5/8
]4/5
for RePr40.2 for average; Tfm
Correlations for internal flow in tubes
Nu¼4.36 for constant surface heat flux; fully developed; laminar
Nu¼3.66 for constant surface temperature; fully developed; laminar
Nu¼3.66 þ (0.065(D/L) Re Pr)/(1 þ 0.04[(D/L) Re Pr)]2/3
for constant surface temperature; developing flow; laminar
Nu¼0.023Re4/5
Prn
for 0.7rPrr160; turbulent; fully developed; ReZ10,000; n¼0.4 for fluid heating; n¼0.3 for fluid
cooling
Nu¼4.8 þ 0.0156Re0.85
Prs
0.93
for liquid metal flow; constant surface temperature; 104
oReo106
Nu¼6.3 þ 0.0167Re0.85
Prs
0.93
for liquid metal flow; constant surface heat flux; 104
oReo106
Correlations for external cross-flow over spheres
Nu/Pr1/3
¼0.37Re0.6
/Pr1/3
for average; Tfm; 17oReo70,000
Nu¼2 þ (0.4Re1/2
þ 0.06Re2/3
)Pr0.4
(ma/ms)1/4
for 0.71oPro380
for average; Ta; 3.5oReo7.6 104
; 1o(ma/ms)o3.2.
Correlation for falling drop
Nu¼2 þ 0.6Re1/2
Pr1/3
[25(x/D)0.7
] for average; Ta
Source: Reproduced from Dincer I. Heat transfer in food cooling applications. Washington, DC: Taylor Francis; 1997.
Air
Ta = 22°C
U = 8.3 m s−1
3m
Ts = 50°C
Fig. 20 Heat transfer from a flat plate solar collector.
Heat Transfer Aspects of Energy 447](https://image.slidesharecdn.com/13comprehensiveenergysystems-240412072703-d37af1a8/75/Comprehensive-energy-systems-pdf-Comprehensive-energy-systems-pdf-26-2048.jpg)
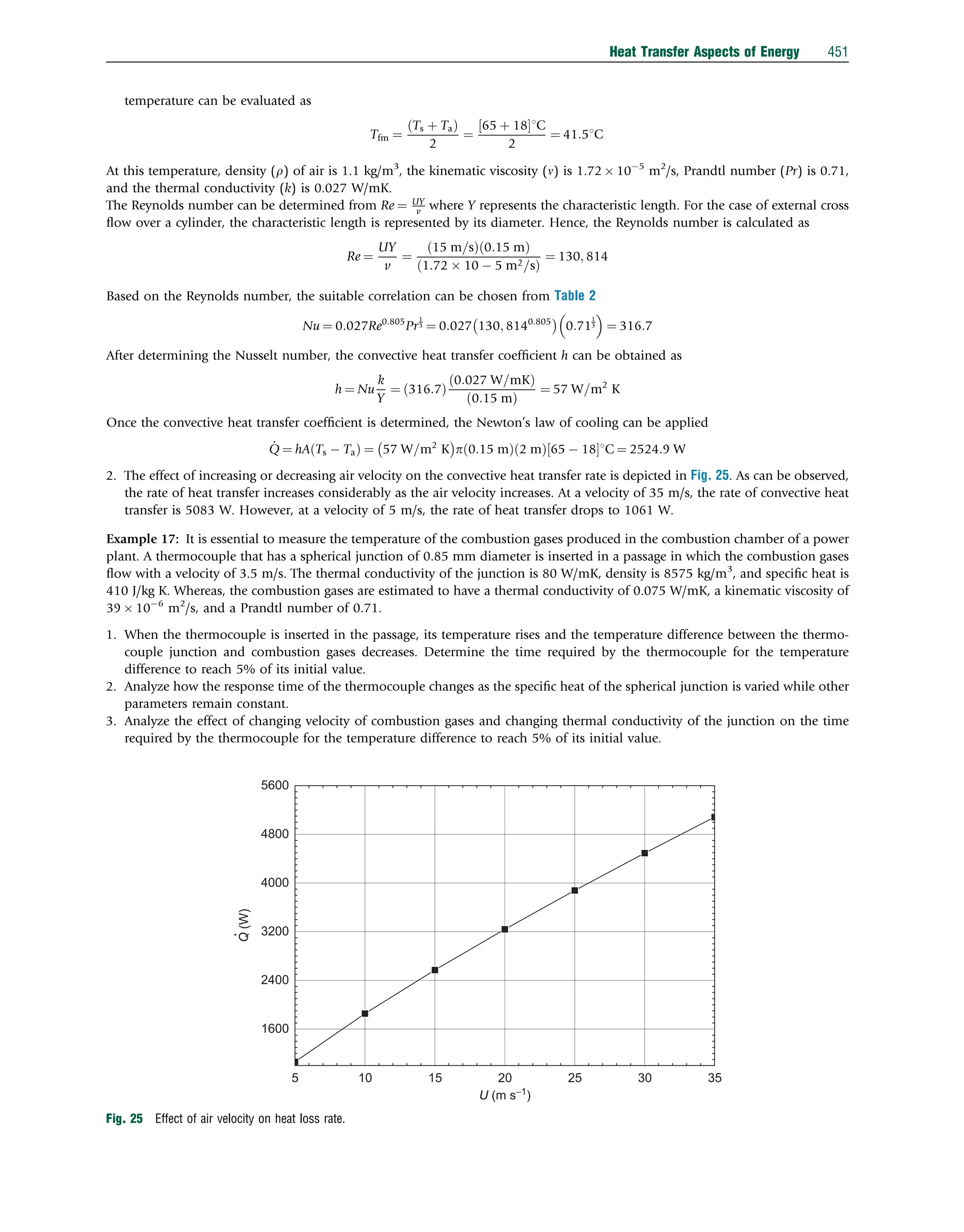



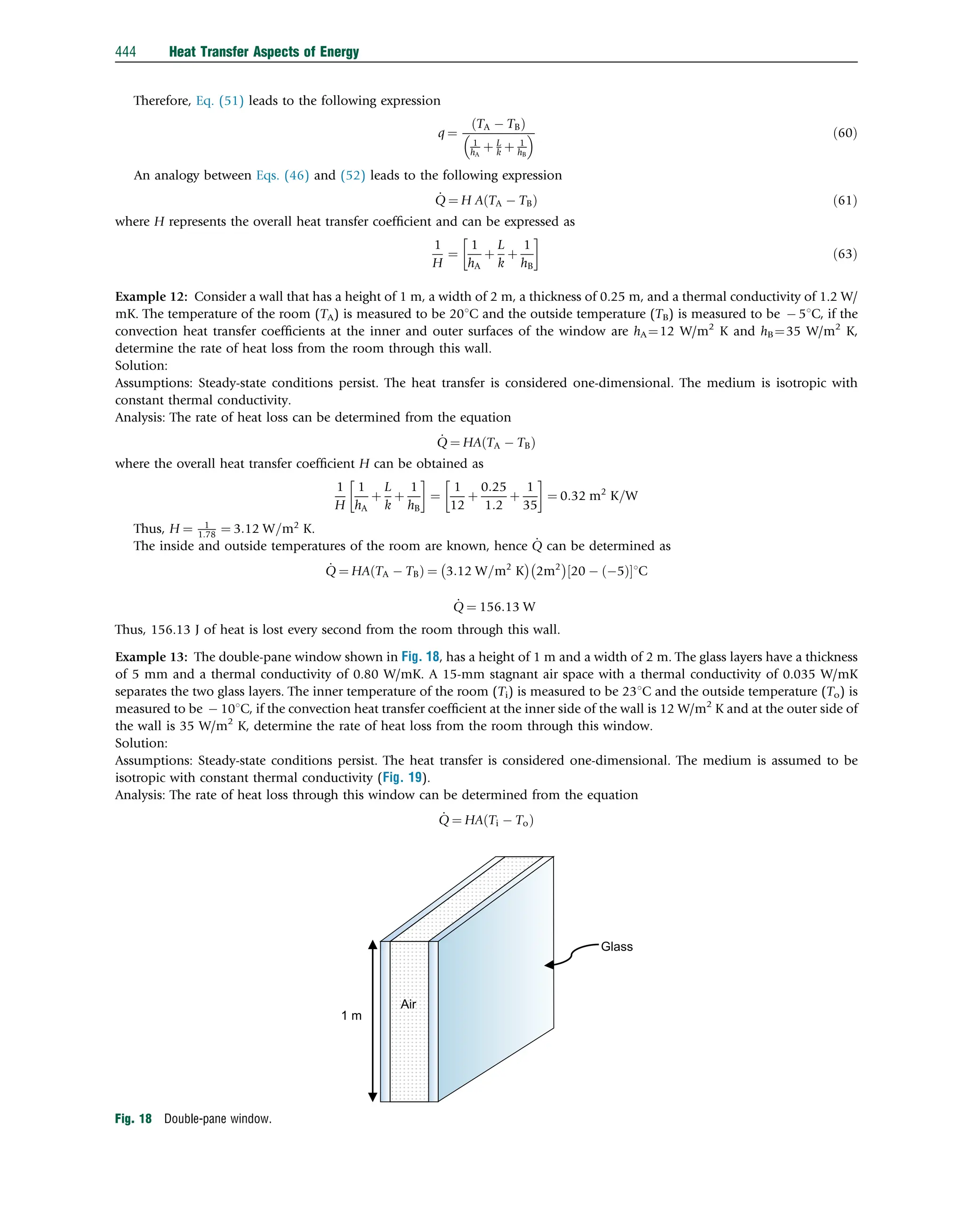
![Example 22: Consider a flat plate solar collector. The collector is placed with a tilt angle of 45 degree and has dimensions
0.5 m 0.5 m. The absorber plate and the glass cover are positioned 2 cm apart. If the temperatures of the glass cover and the
absorber plate are measured to be 651C and 881C, respectively, determine the rate of heat loss due to natural convection from the
absorber plate of the collector (Fig. 35).
Solution: The mean temperature can be obtained as Tm ¼ 65þ88
½ 1C
2 ¼ 76:51C. The properties of air are obtained at this mean
temperature. The thermal conductivity k is 0:029 W=m2
K, kinematic viscosity n is 2.1 105
m2
/s, thermal diffusivity a is
2.9 105
m2
/s, and coefficient of volume expansion b is 1
273þ76:5
½ K ¼ 0:0029.
After obtaining the properties, the Rayleigh number can be determined
Ra ¼
gbDTL3
va
¼
9:81 m=s2
ð Þ 0:0029 1=K
ð Þ 231C
ð Þ 0:021m
ð Þ3
2:1 105
m2=s
ð Þ 2:9 105
m2=s
ð Þ
¼ 8480:5
Table 5 Natural convection heat transfer equations and correlations
Equation or correlation
General equations
Nu¼hY/kf ¼cRan
and Ra¼Gr Pr¼gb(Ts Ta)Y3
/na
where n is 1/4 for laminar flow and 1/3 for turbulent flow. Y is height for vertical plates or pipes, diameter for horizontal pipes and
spheres Tfm(Ts þ Ta)/2
Correlations for vertical plates (or inclined plates, inclined up to 60 degree)
Nu¼[0.825 þ 0.387Ra1/6
/(1 þ (0.492/Pr)9/16
)4/9
]2
for an entire range of Ra
Nu¼0.68 þ 0.67Ra1/4
/(1 þ (0.492/Pr)9/16
)4/9
for 0oRao109
Correlations for horizontal plates (YAs/P)
For upper surface of heated plate or lower surface of cooled plate:
Nu¼0.54Ra1/4
for 104
rRar107
Nu¼0.15Ra1/3
for 107
rRar1011
For lower surface of heated plate or upper surface of cooled plate:
Nu¼0.27Ra1/4
for 105
rRar1010
Correlations for horizontal cylinders
Nu¼hD/k¼cRan
where
c¼0.675 and n¼0.058 for 1010
oRao102
c¼1.020 and n¼0.148 for 102
oRao102
c¼0.850 and n¼0.188 for 102
oRao104
c¼0.480 and n¼0.250 for 104
oRao107
c¼0.125 and n¼0.333 for 107
oRao1012
Nu¼[0.60 þ 0.387Ra1/6
/(1 þ (0.559/Pr)9/16
)8/27
]2
for an entire range of Ra
Correlations for spheres
Nu¼2 þ [0.589Ra1/4
/(1 þ (0.469/Pr)9/16
)4/9
] for PrZ0.7 and Rar1011
Correlations for enclosures
Rectangular inclined enclosures
Nu¼1 þ 1.44[1–(1708/Ra cosy)]þ
(1 1708 (sin 1.8y)1.6
/Ra cosy) þ [(Ra cosy)1/3
/18) 1]þ
Vertical rectangular enclosures
Nu¼0.18[Ra (Pr/(0.2 þ Pr))]0.29
for 1oH/Lo2; RaPr/(0.2 þ Pr)4103
Nu¼0.22[Ra (Pr/(0.2 þ Pr))]0.28
(H/L) 1/4
for 2oH/Lo10; Rao1010
Nu¼0.42Ra1/4
Pr0.012
(H/L) 0.3
for 10oH/Lo40; 1oPro2 104
; 104
oRao107
Nu¼0.046Ra1/3
for 1oH/Lo40; 1oPro20; 106
oRao109
Concentric cylinders
keff/k¼0.386(Pr/(0.861 þ Pr))1/4
(Fc Ra)1/4
Heat transfer correlations
Gr Pr¼1.6 106
Y3
(DT)
where Y in m; DT in 1C
h¼0.29 (DT/Y)1/4
for vertical small plates in laminar range
h¼0.19 (DT)1/3
for vertical large plates in turbulent range
h¼0.27 (DT/Y)1/4
for horizontal small plates in laminar range (facing upward when heated or downward when cooled)
h¼0.22 (DT)1/3
for vertical large plates in turbulent range (facing downward when heated or upward when cooled)
h¼0.27 (DT/Y)1/4
for small cylinders in laminar range
h¼0.18 (DT)1/3
for large cylinders in turbulent range
Source: Reproduced from Dincer I. Heat transfer in food cooling applications. Washington, DC: Taylor Francis; 1997.
460 Heat Transfer Aspects of Energy](https://image.slidesharecdn.com/13comprehensiveenergysystems-240412072703-d37af1a8/75/Comprehensive-energy-systems-pdf-Comprehensive-energy-systems-pdf-39-2048.jpg)








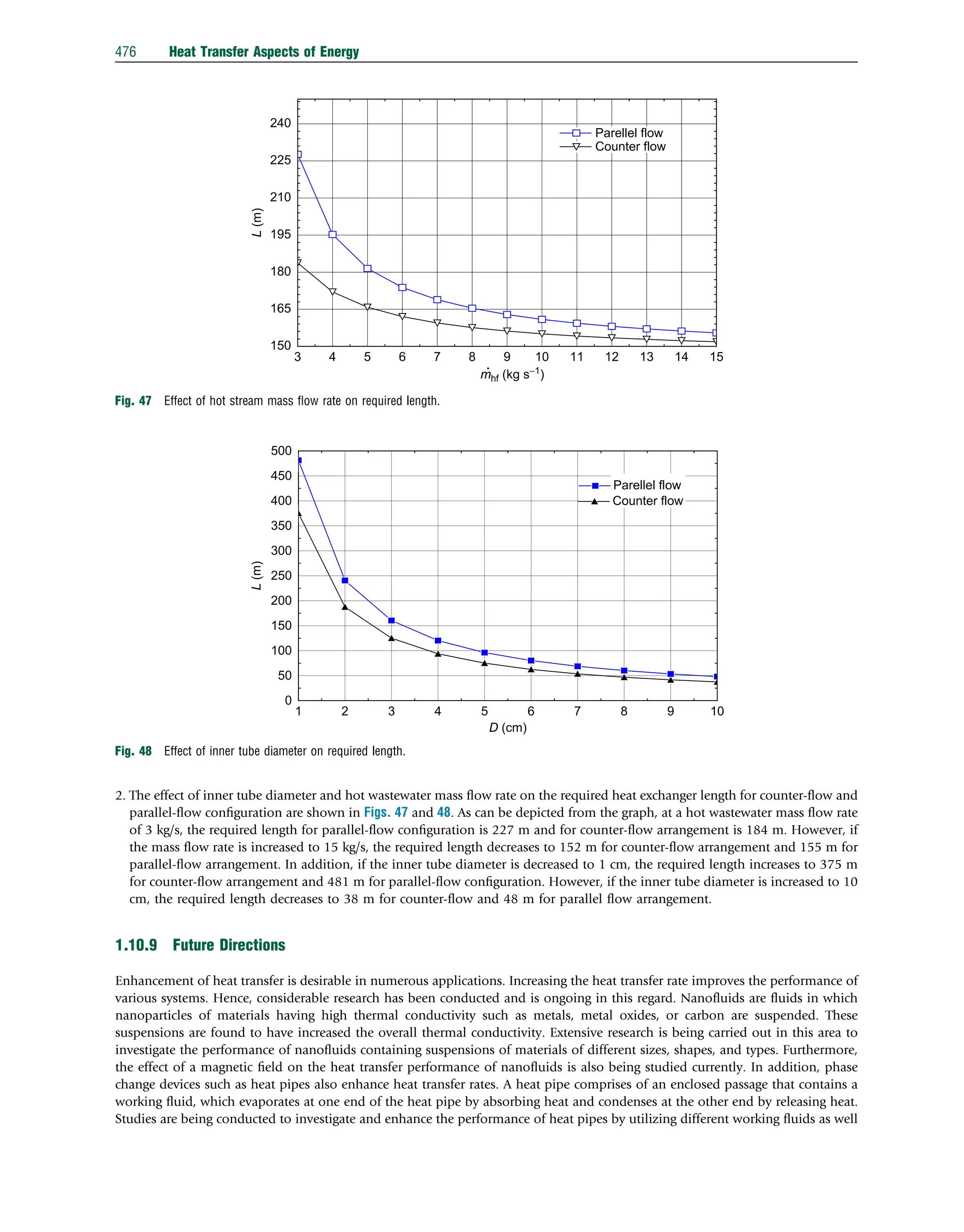
![as size and shape of heat pipes in different applications. Moreover, composite materials with significantly high thermal con-
ductivity are being investigated for the enhancement of heat transfer in different applications. Various compositions and materials
are being studied in this regard. Extended surfaces such as fins are used extensively in various applications to enhance the heat
transfer rate. Numerous studies are being conducted to investigate the performance of different types, shapes, configurations,
combinations, and materials of fins for different applications. Furthermore, the effect and characteristics of vortex generators with
regard to heat transfer are also being investigated.
1.10.10 Conclusions
This chapter is focused on the heat transfer aspects of energy. The chapter first discusses the first law of thermodynamics and energy
balance, which is the underlying principle for heat transfer analysis. Further, the analysis of different modes of heat transfer for
different types of cases and geometries is discussed. Heat transfer analysis forms an integral part of different aspects of energy
applications. The chapter relates the analysis of heat transfer to different energy applications. For instance, heat transfers though
walls or windows, which are essential to determine the heating or cooling load of a building, are discussed and analyzed.
Furthermore, determining the rate of heat loss from solar collectors, which is essential to analyze their performance, is also
discussed. In addition, analysis of heat exchangers, which is essential in numerous applications, is also briefly described. Analyzing
the rate of heat loss or gain by a given system is vital to study its overall performance.
References
[1] Dincer I. Analytical modelling of heat transfer from a single slab in freezing. Int J Energy Res 1995;19(3):227–33.
[2] Dincer I. Transient temperature distributions in spherical and cylindrical food products subjected to hydrocooling. Int J Energy Res 1994;18(8):741–9.
Further Reading
Cengel YA, Boles MA. Thermodynamics: an engineering approach. Boston: McGraw-Hill; 2002.
Cengel YA, Ghajar AJ. Heat and mass transfer: fundamentals applications. New York, NY: McGraw-Hill Education; 2015.
Dincer I. Thermodynamics, exergy and environmenptal impact. In: Proceedings of the ISTP-11, the eleventh international symposium on transport phenomena, Hsinchu, Taiwan;
1998. p.121–125, 29 November–3 December, 1998.
Duffie JA, Beckman WA. Solar engineering of thermal processes. Hoboken, NY: Wiley; 2013.
Relevant Websites
https://www.asme.org/engineering-topics/heat-transfer
American Society of Mechanical Engineers.
https://www.britannica.com/science/heat-transfer
Encyclopedia Britannica.
https://www.thermalfluidscentral.org/encyclopedia/index.php/Main_Page
Thermal-FluidsPedia.
Heat Transfer Aspects of Energy 477](https://image.slidesharecdn.com/13comprehensiveenergysystems-240412072703-d37af1a8/75/Comprehensive-energy-systems-pdf-Comprehensive-energy-systems-pdf-56-2048.jpg)