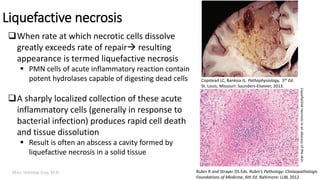
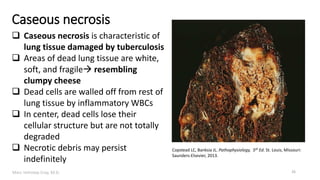
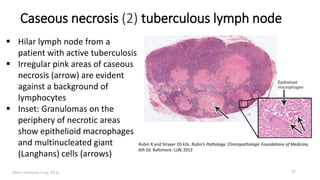
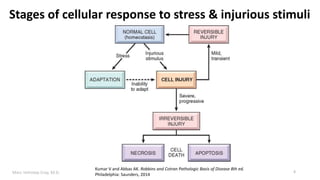
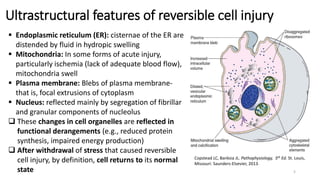
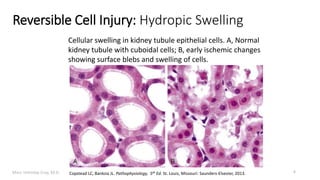
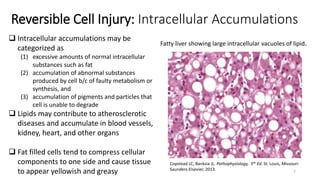
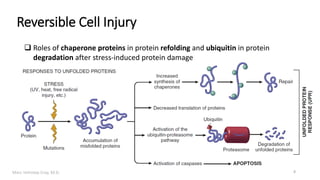
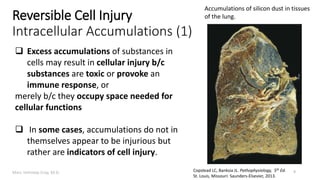
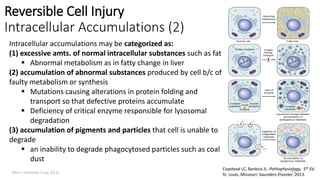
This document provides an overview of cell injury and cell death processes presented by Dr. Marc Imhotep Cray. It discusses reversible cell injury mechanisms including hydropic swelling, intracellular accumulations, and cellular adaptation processes. It also covers irreversible cell injury mechanisms of necrosis and apoptosis. Necrosis types such as coagulative, liquefactive, caseous, and fat necrosis are described. The document provides histological images and discusses the cellular and molecular mechanisms involved in different types of cell injury and death.









![Marc Imhotep Cray, M.D.
Mechanisms by which ischemia leads to
cellular death by necrosis
23
1. Loss of oxygen due to vascular occlusion
impairs mitochondrial function resulting
in decreased energy (adenosine triphosphate
[ATP]) production by aerobic processes
2. Decreased ATP impairs ATP-dependent ion
exchangers
3. Loss of aerobic processes causes anaerobic
glycolysis to predominate, with consequent
intracellular acidosis, eventually leading to
increased cytosolic [Ca2+]
4. Ca2+-dependent phospholipases are then
activated, causing loss of cell membrane
integrity and necrosis
Rubin R and Strayer DS Eds. Rubin’s Pathology: Clinicopathologic
Foundations of Medicine, 6th Ed. Baltimore: LLW, 2012](https://image.slidesharecdn.com/cellinjuryandcelldeath-161006032449/85/Cell-Injury-and-Cell-Death-23-320.jpg)