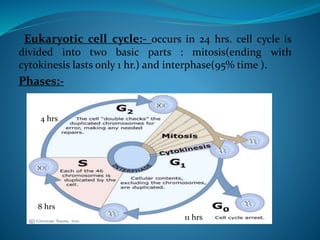
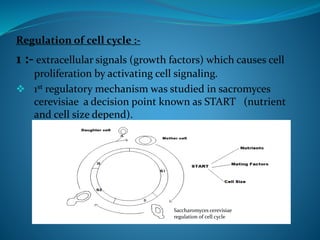
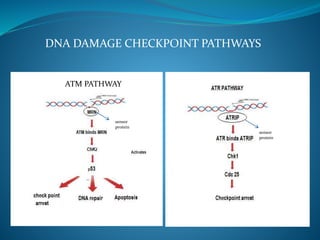
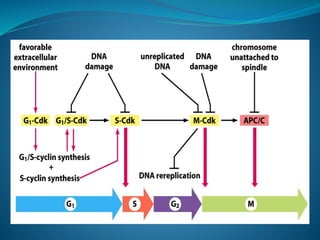
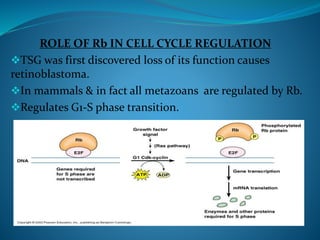
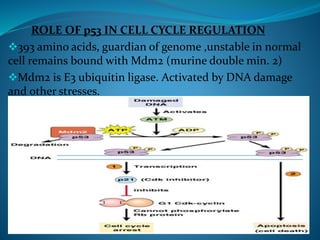
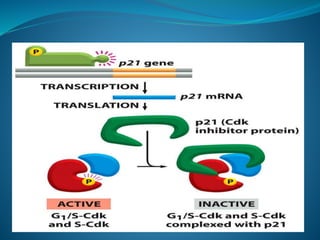
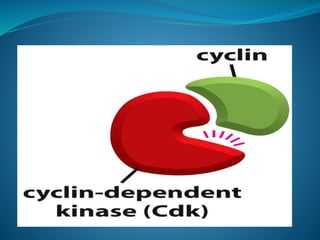
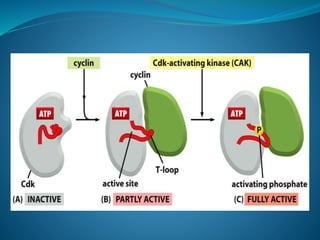
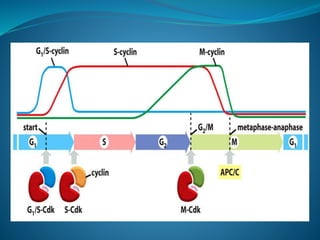
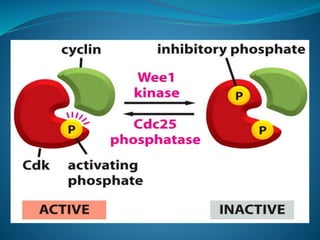
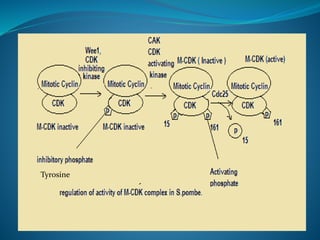
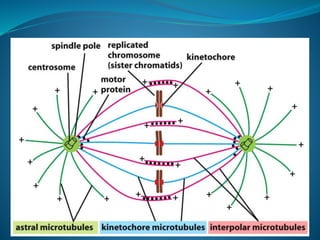
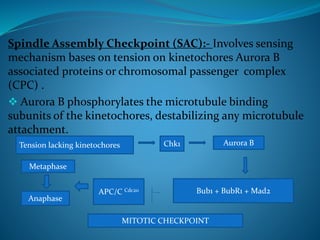
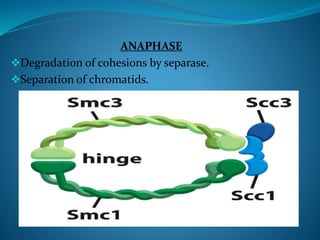
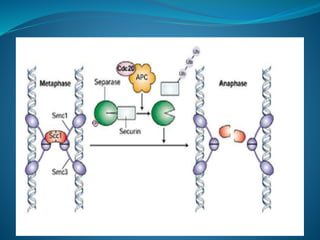
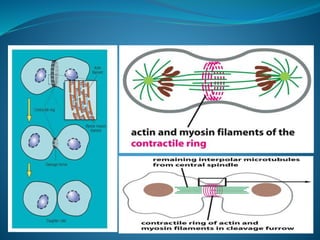
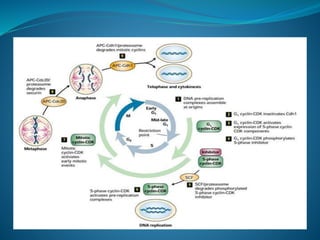
This document summarizes key aspects of the cell cycle and its regulation. It describes the main phases of the eukaryotic cell cycle including interphase and mitosis. Checkpoints that ensure DNA replication fidelity like ATM/ATR pathways are discussed. Central regulators of the cell cycle like cyclins, CDKs, and CDK inhibitors are covered. The roles of Rb and p53 tumor suppressors are mentioned. The stages of mitosis and spindle assembly checkpoint are briefly outlined.