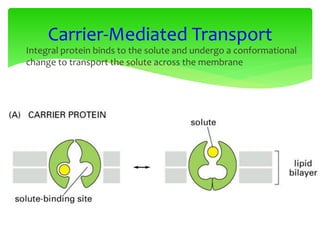
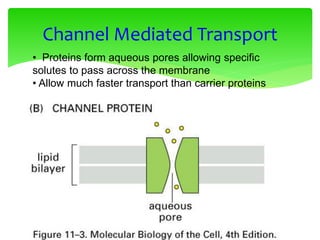
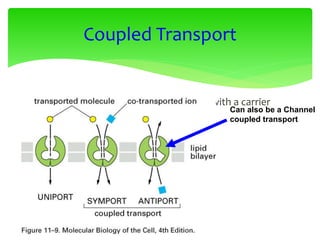
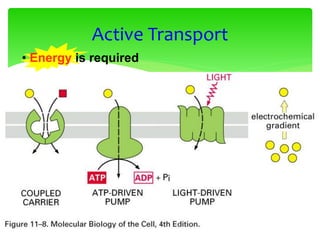
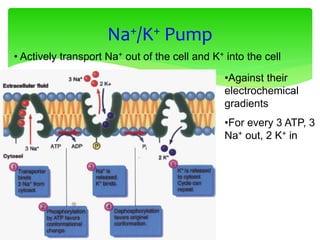
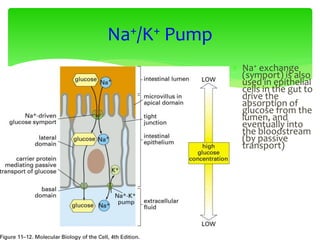
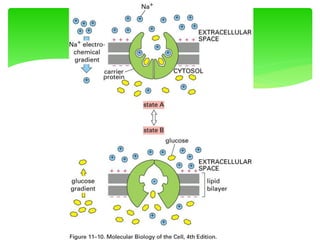
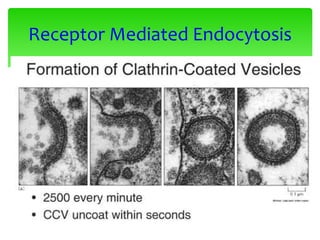
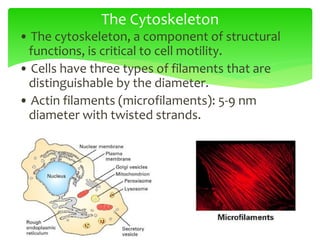
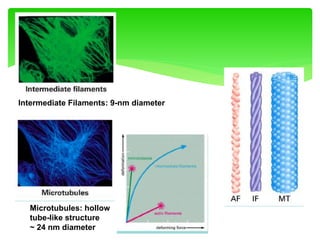
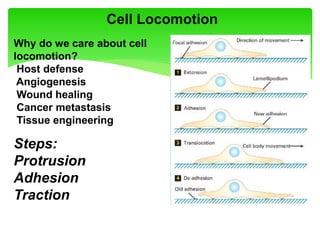
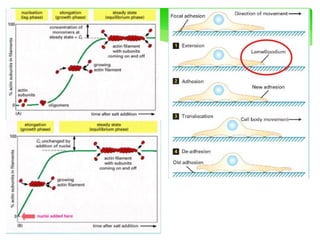
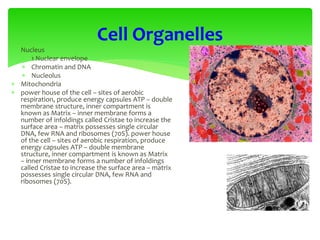
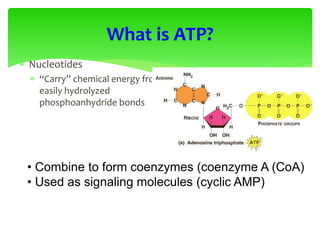
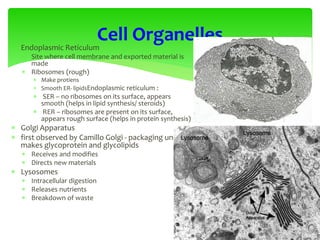
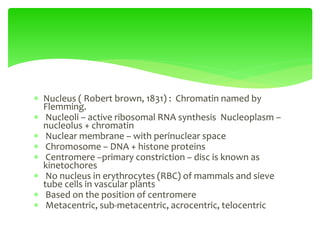
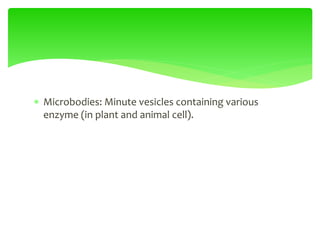
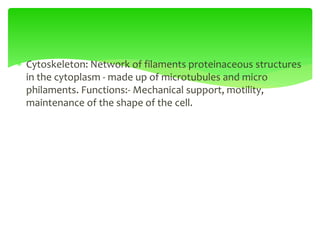
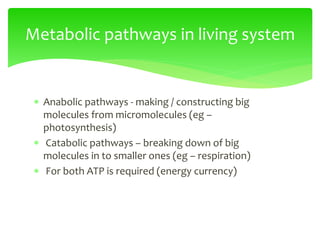
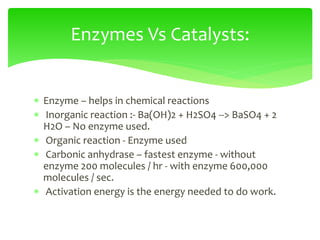
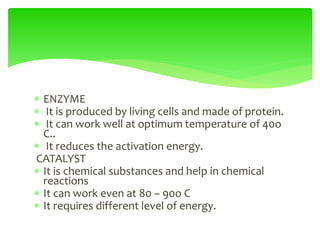
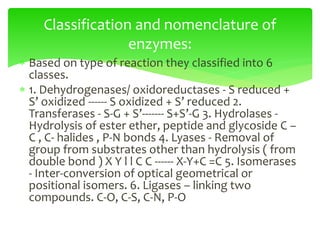
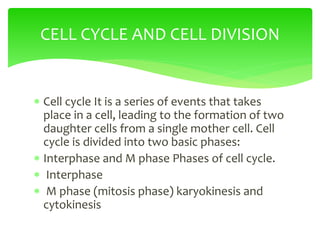
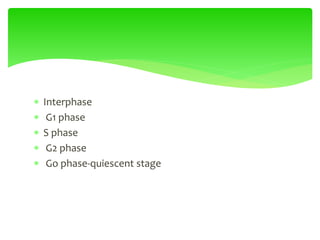
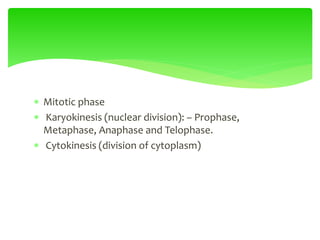
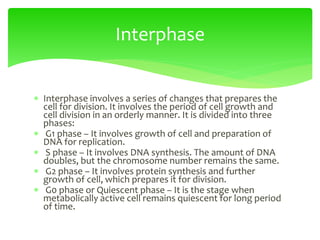
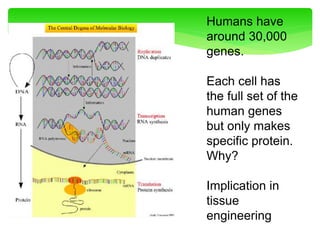
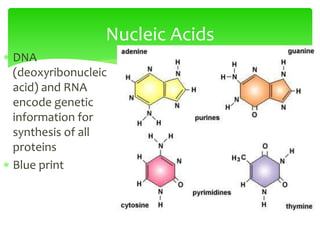
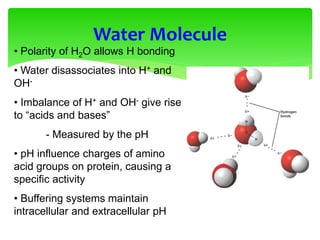
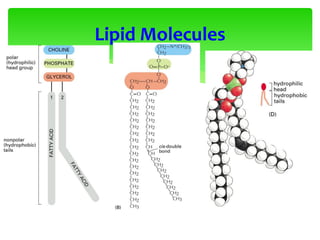
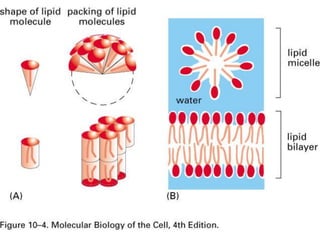
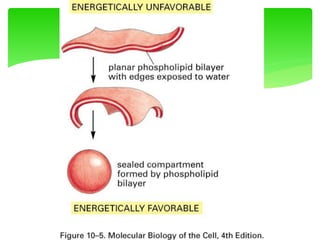
This document provides an overview of cell biology. It begins with an outline of topics covered, including cell structure and organelles, molecular components, properties of cells, and molecule transportation. Key points include: cells being the fundamental unit of life; the discovery of cells by Anton Van Leeuwenhoek and nuclei by Robert Brown; and the cell theory developed by Schleiden, Schwann, and Virchow. The document then discusses the structures and components of prokaryotic and eukaryotic cells. It provides details on various cell organelles and their functions, as well as biomolecules like proteins, nucleic acids, and polysaccharides. Finally, it covers topics such as metabolism, enzymes, and enzyme classification


















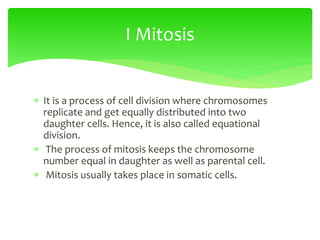










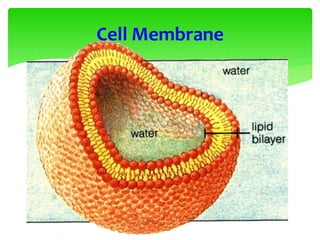

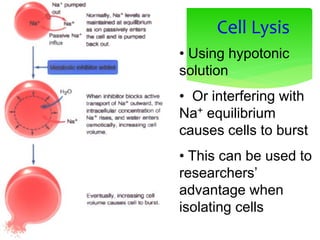
![Erythrocyte Cell
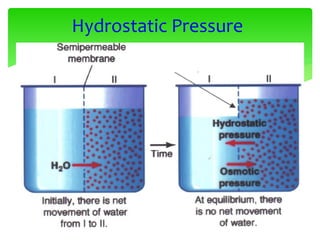
Equilibrium
•No osmotic pressure
- cell is in an isotonic solution
- Water does not cross
membrane
•Increased [Osmotic] in cytoplasm
- cell is in an hypotonic solution
- Water enters cell, swelling
•Decreased [Osmotic] in cytoplasm
- cell is in an hypotonic solution
- Water leaves cell, shrinking](https://image.slidesharecdn.com/cellbiology-171207155014/85/Cell-biology-81-320.jpg)