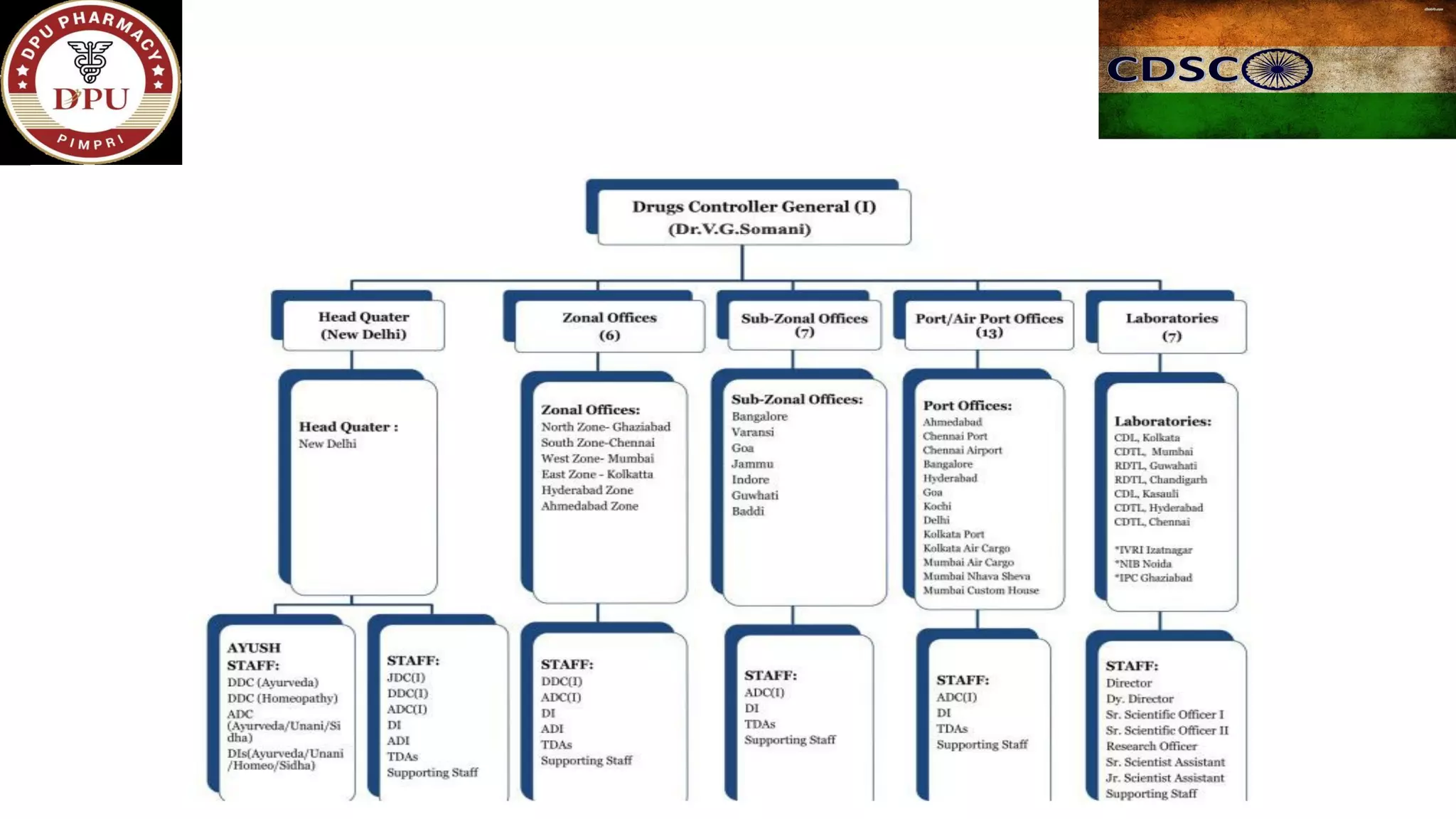
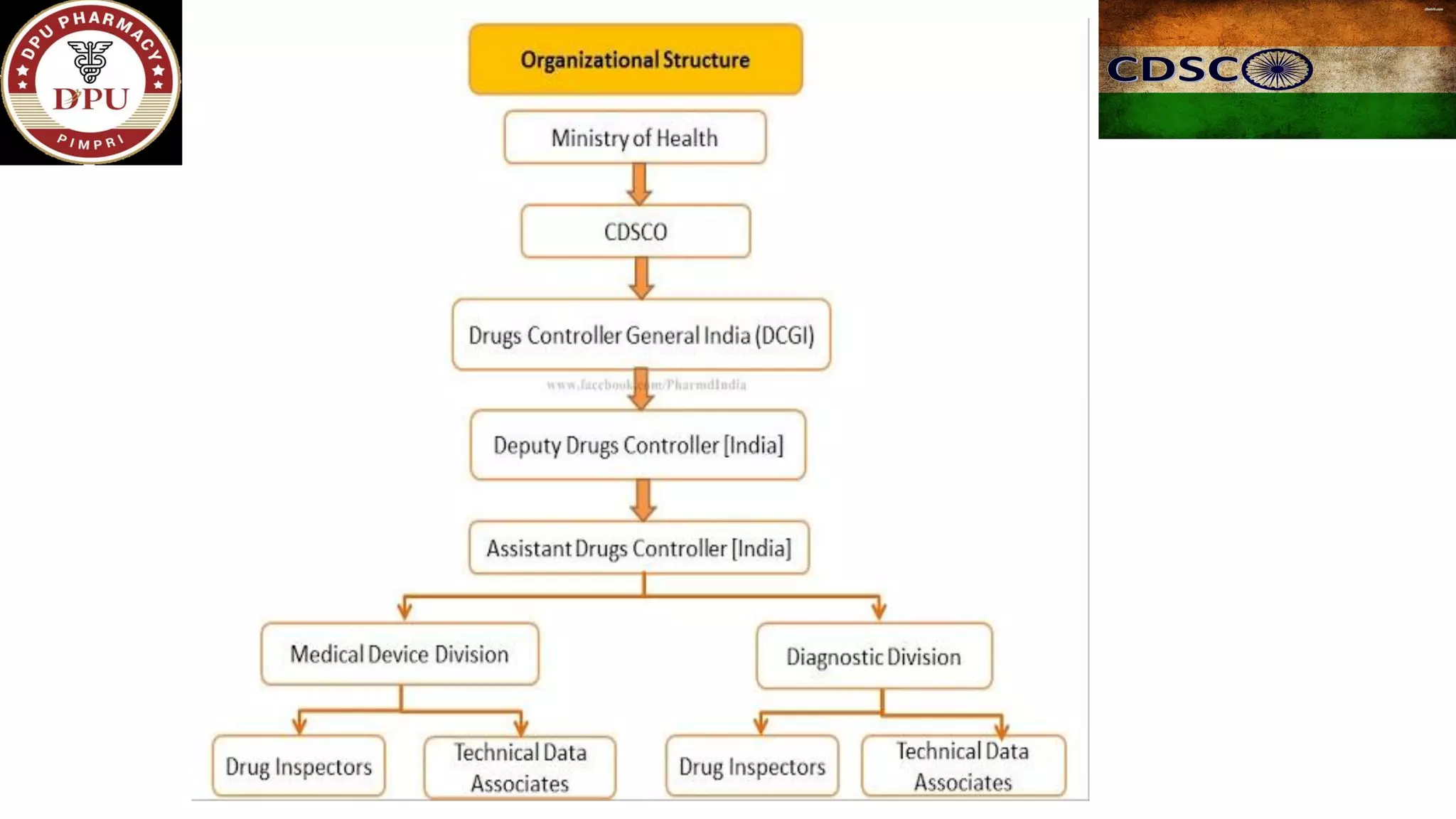
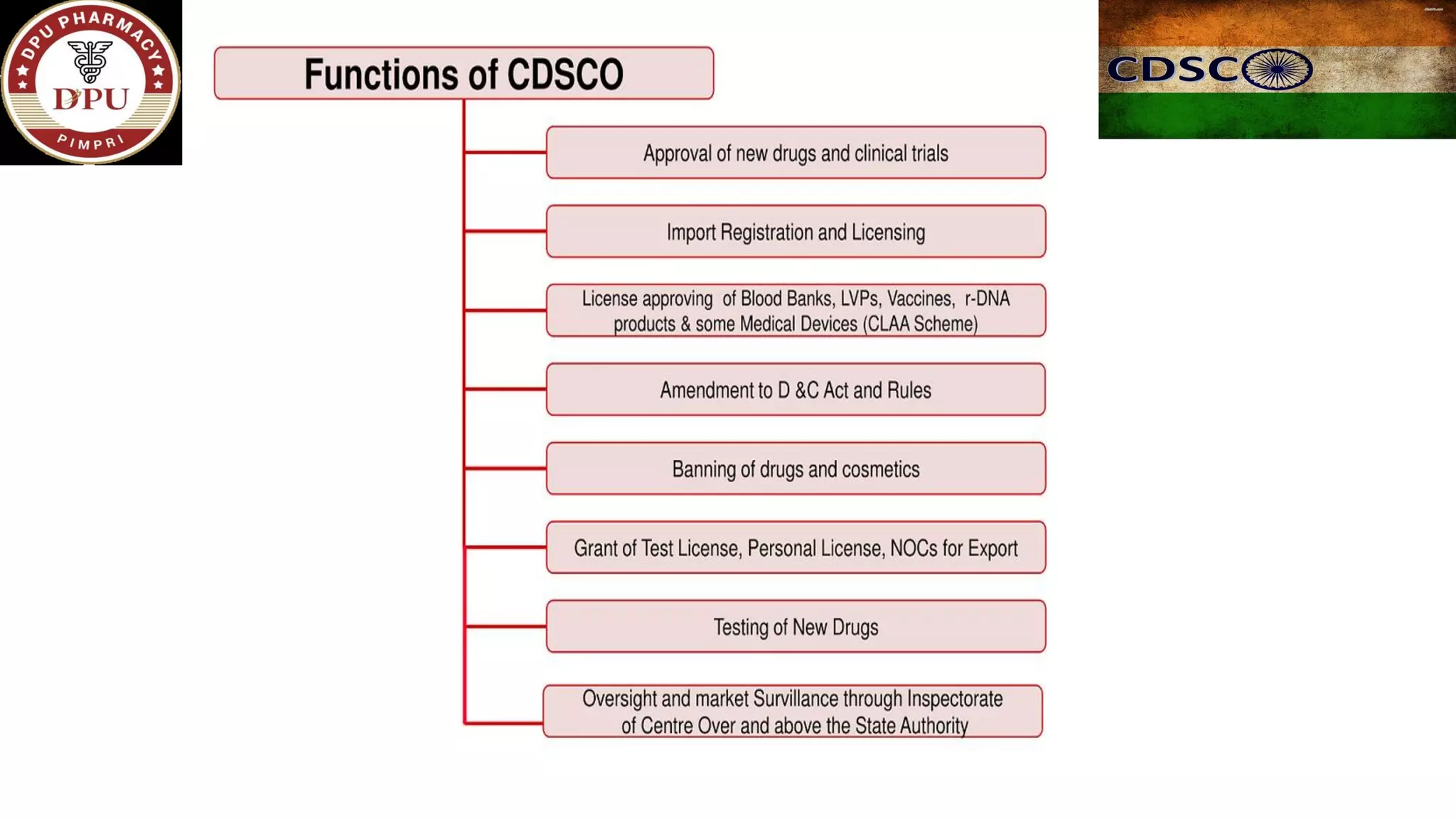
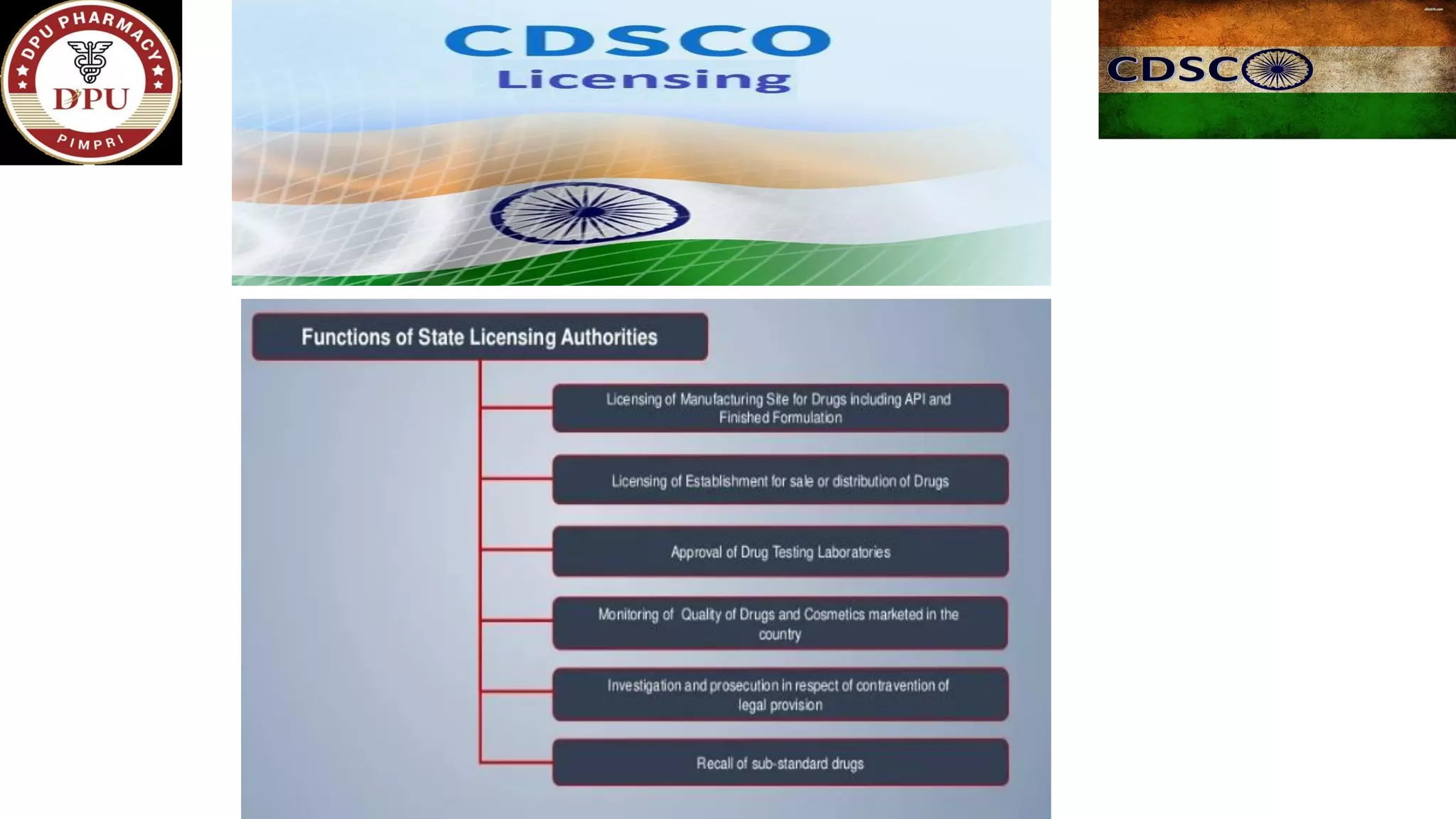
The document provides a comprehensive overview of the Central Drugs Standard Control Organization (CDSCO) in India, detailing its structure, functions, and regulatory guidelines for pharmaceuticals, medical devices, cosmetics, and clinical trials. It describes the CDSCO's role in ensuring safety and efficacy in drug approval processes, the organization of its operations including zonal offices and laboratories, and specific guidelines for various applications including the Common Technical Document (CTD). Additionally, it outlines the import regulations for cosmetics and the classification and approval processes for medical devices and clinical trials.