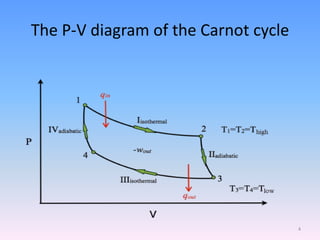
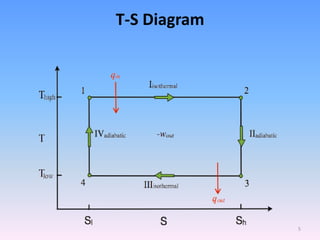
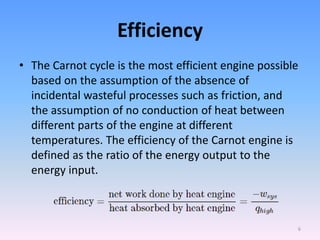
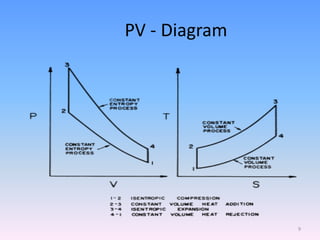
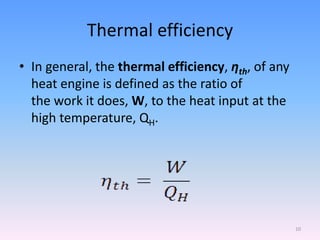
The document compares the Carnot cycle and Otto cycle. The Carnot cycle consists of four processes - two reversible adiabatic expansions and two reversible isothermal compressions. It has the highest possible efficiency. The Otto cycle models the four-stroke internal combustion engine. It consists of intake, compression, power, and exhaust strokes. Like the Carnot cycle, it converts heat into work but is less efficient. Both cycles are important in modeling heat engines.