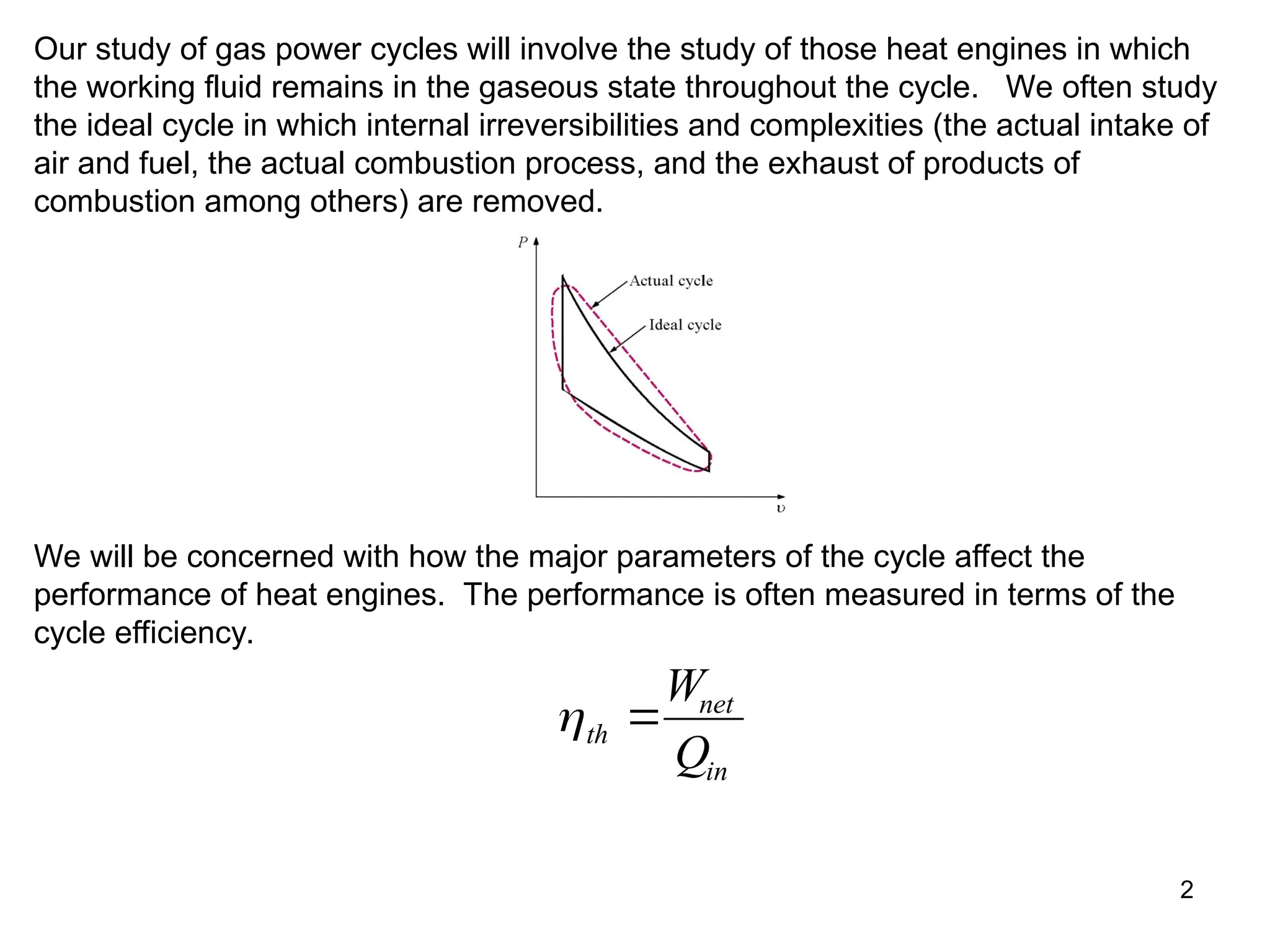
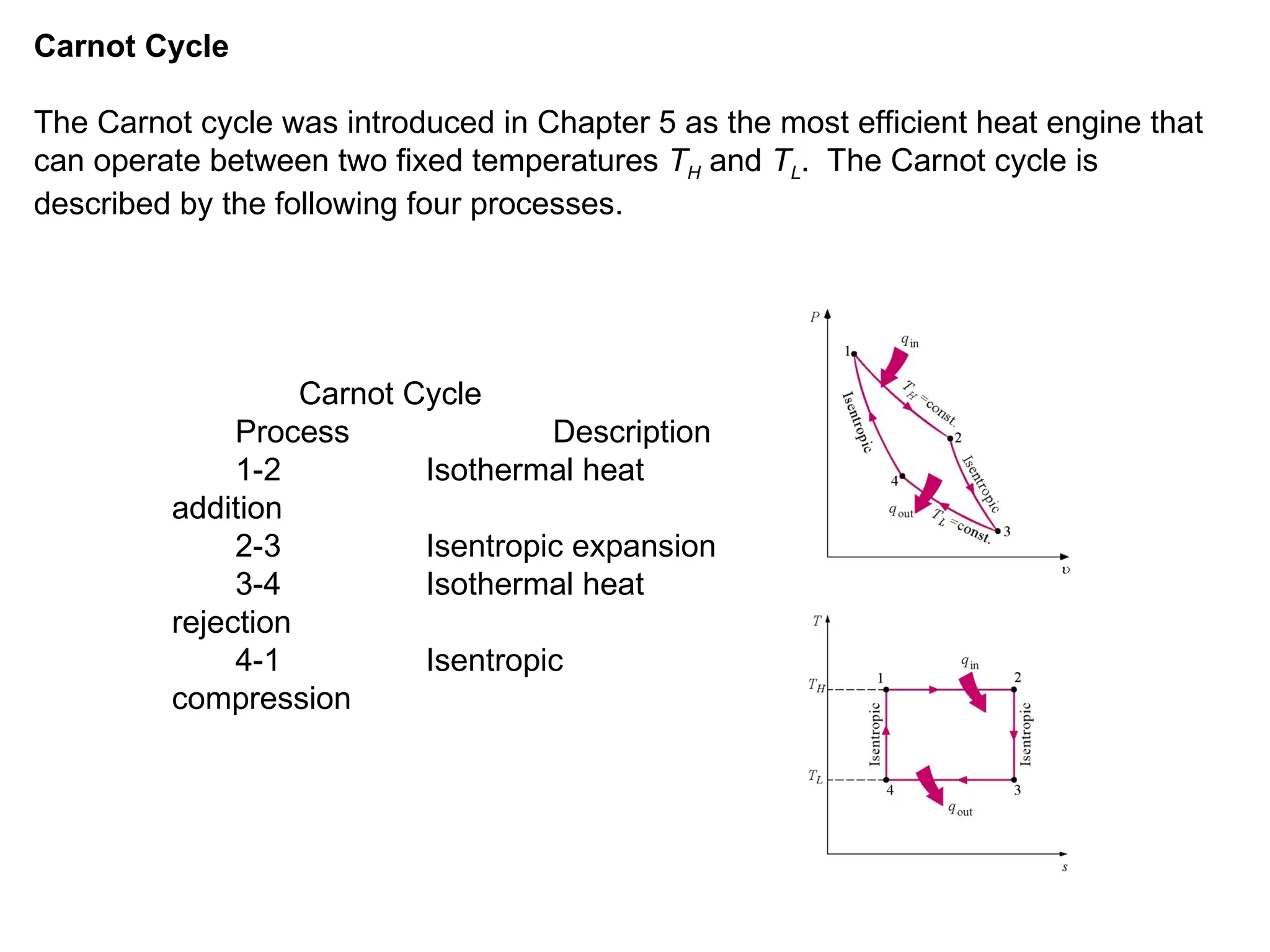
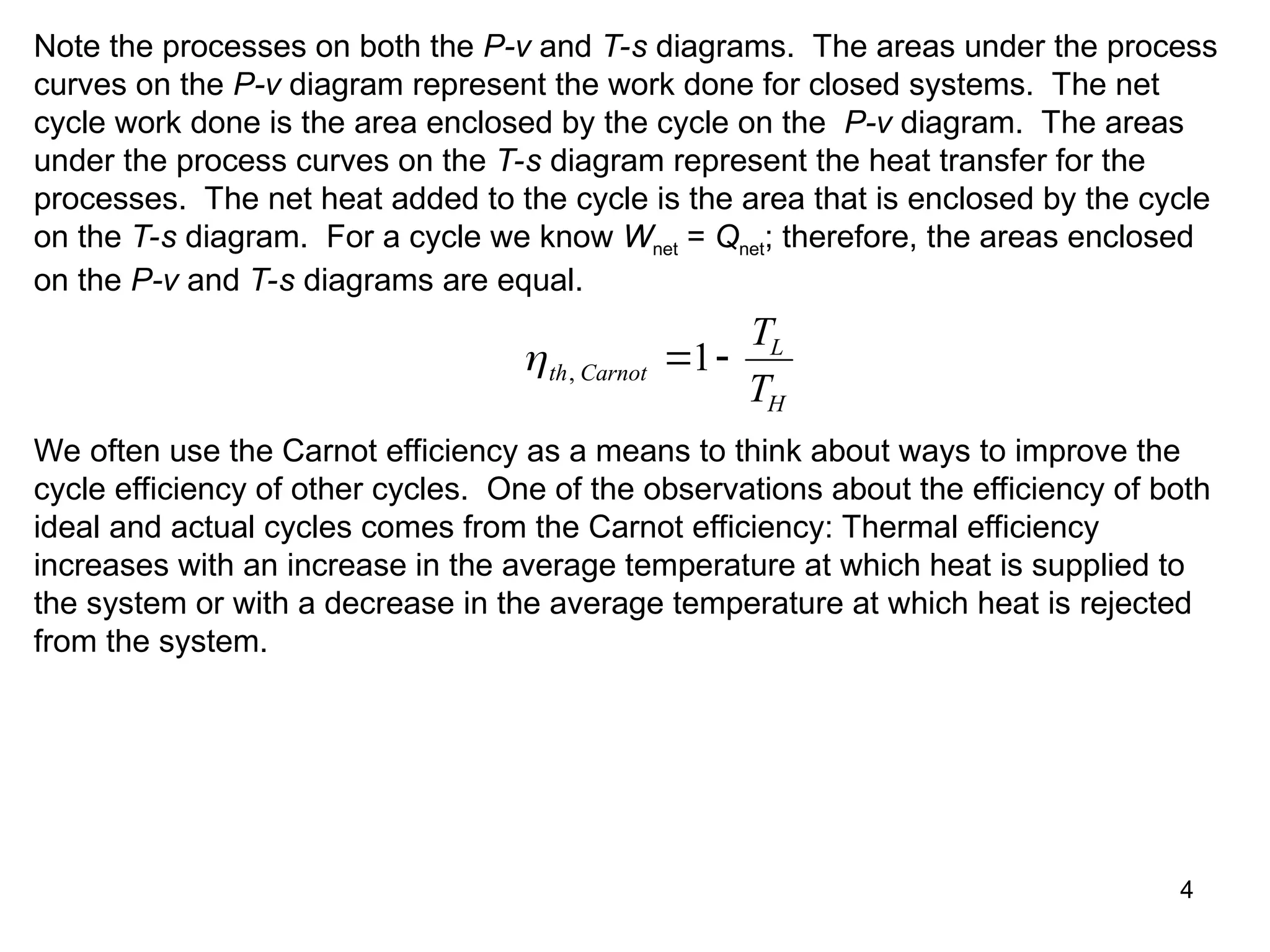
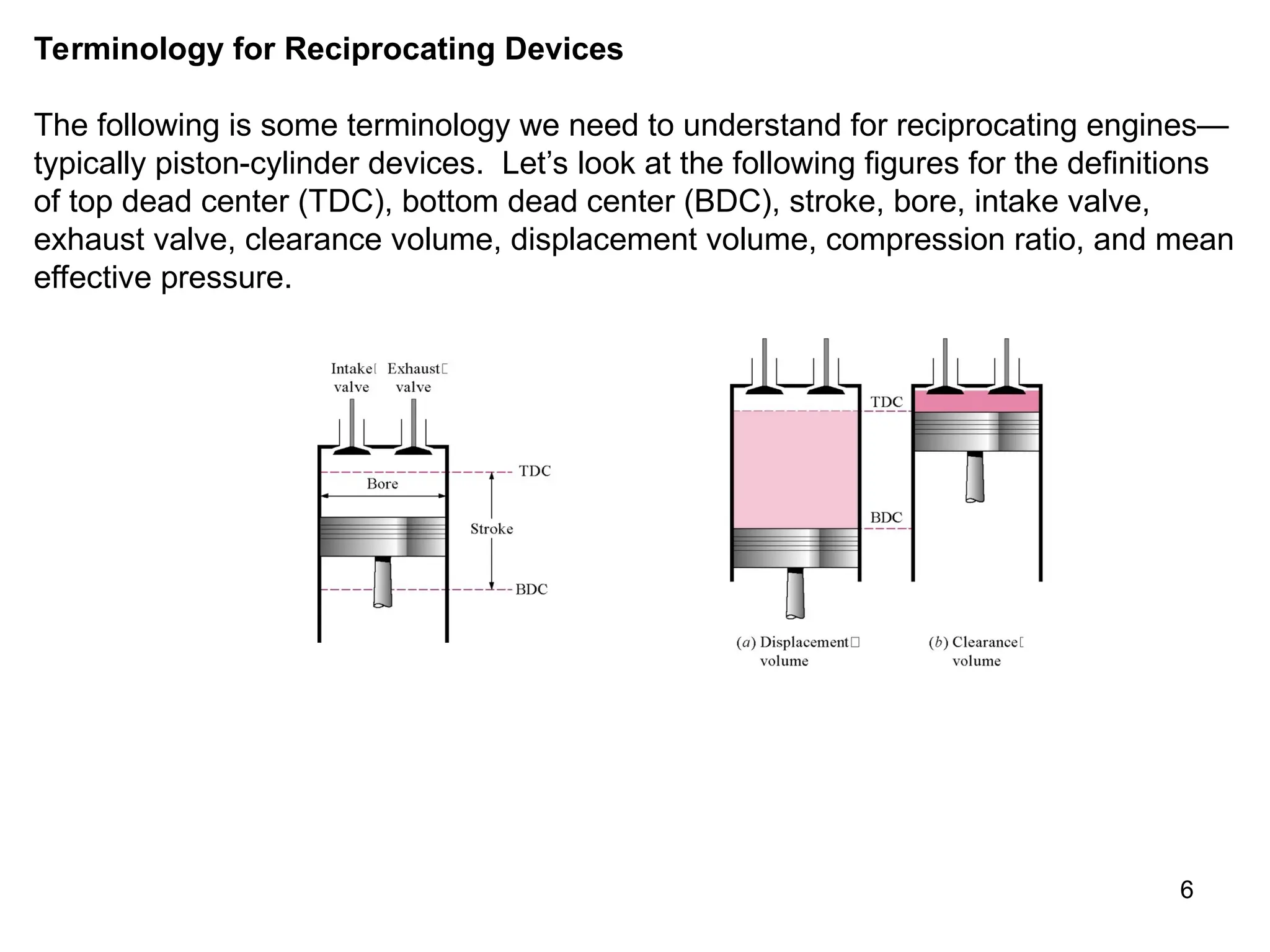
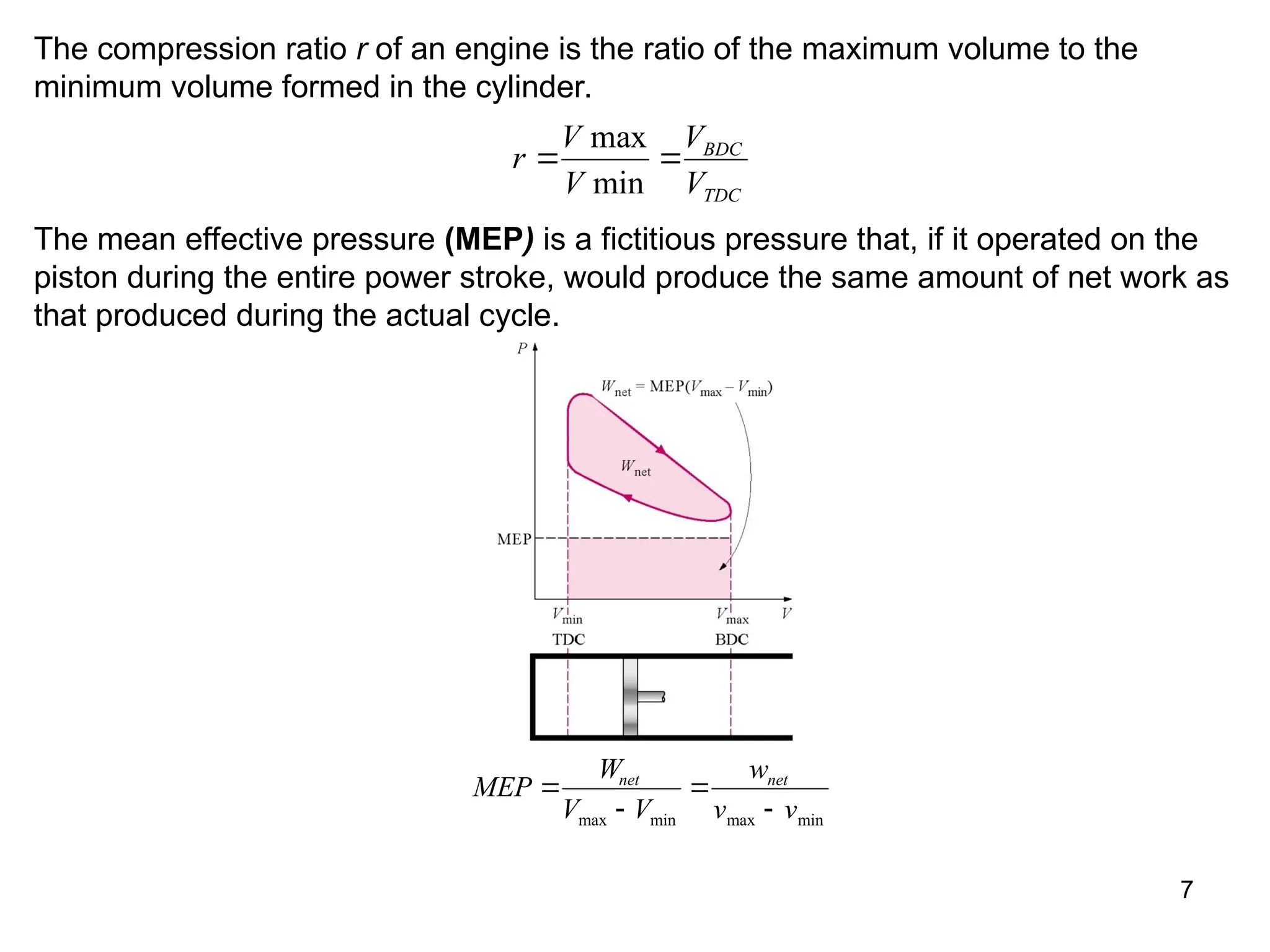
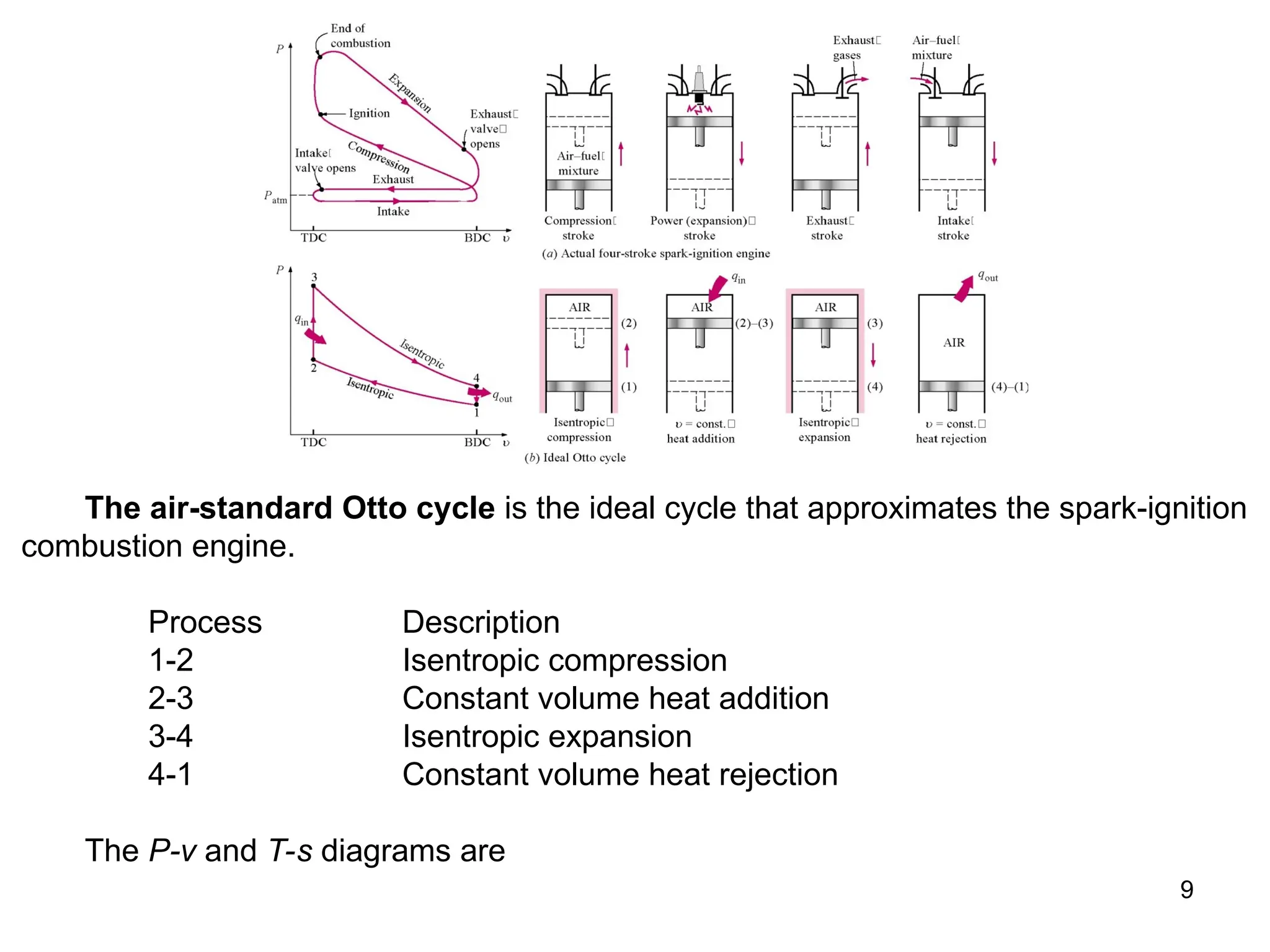
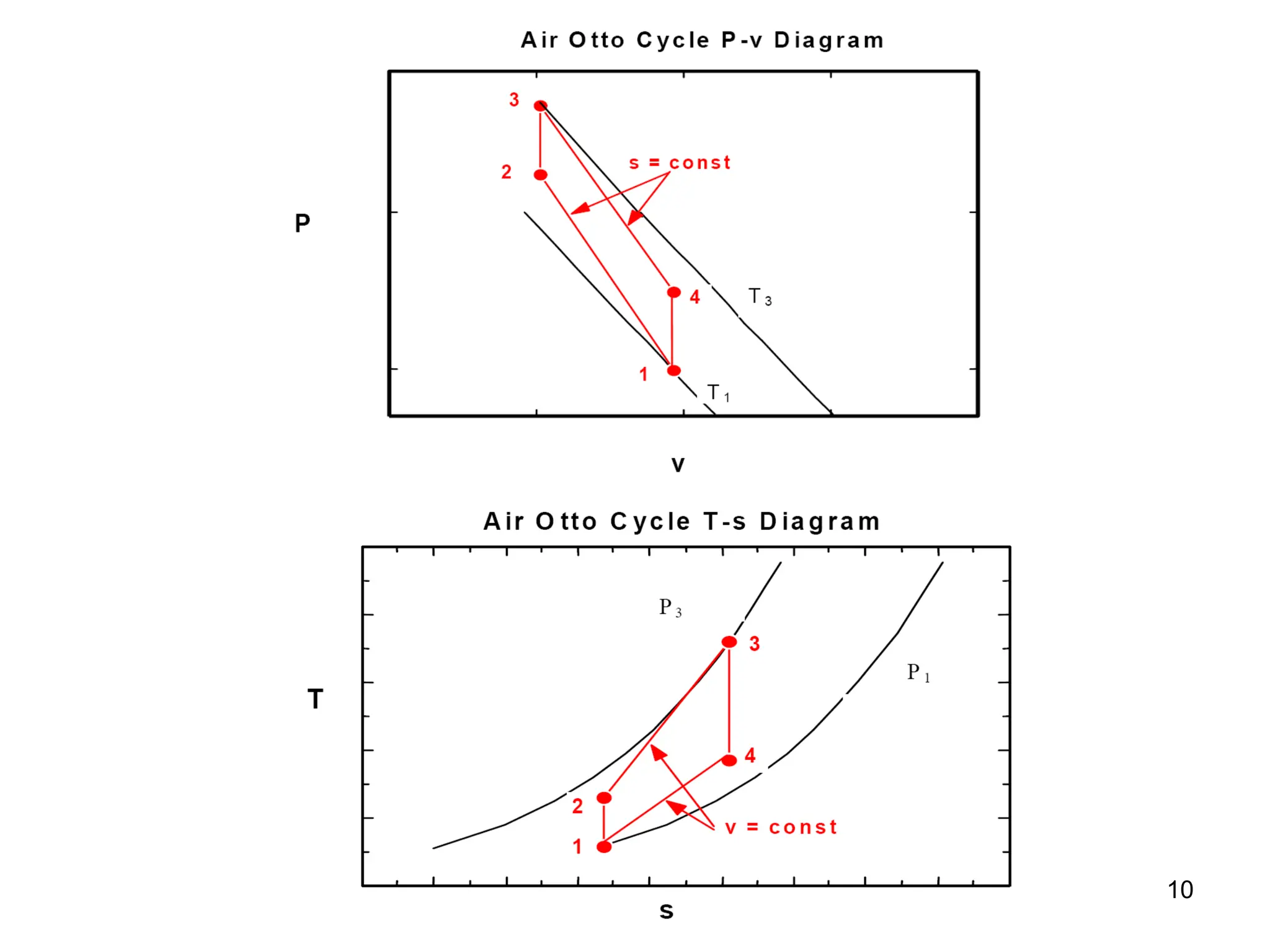
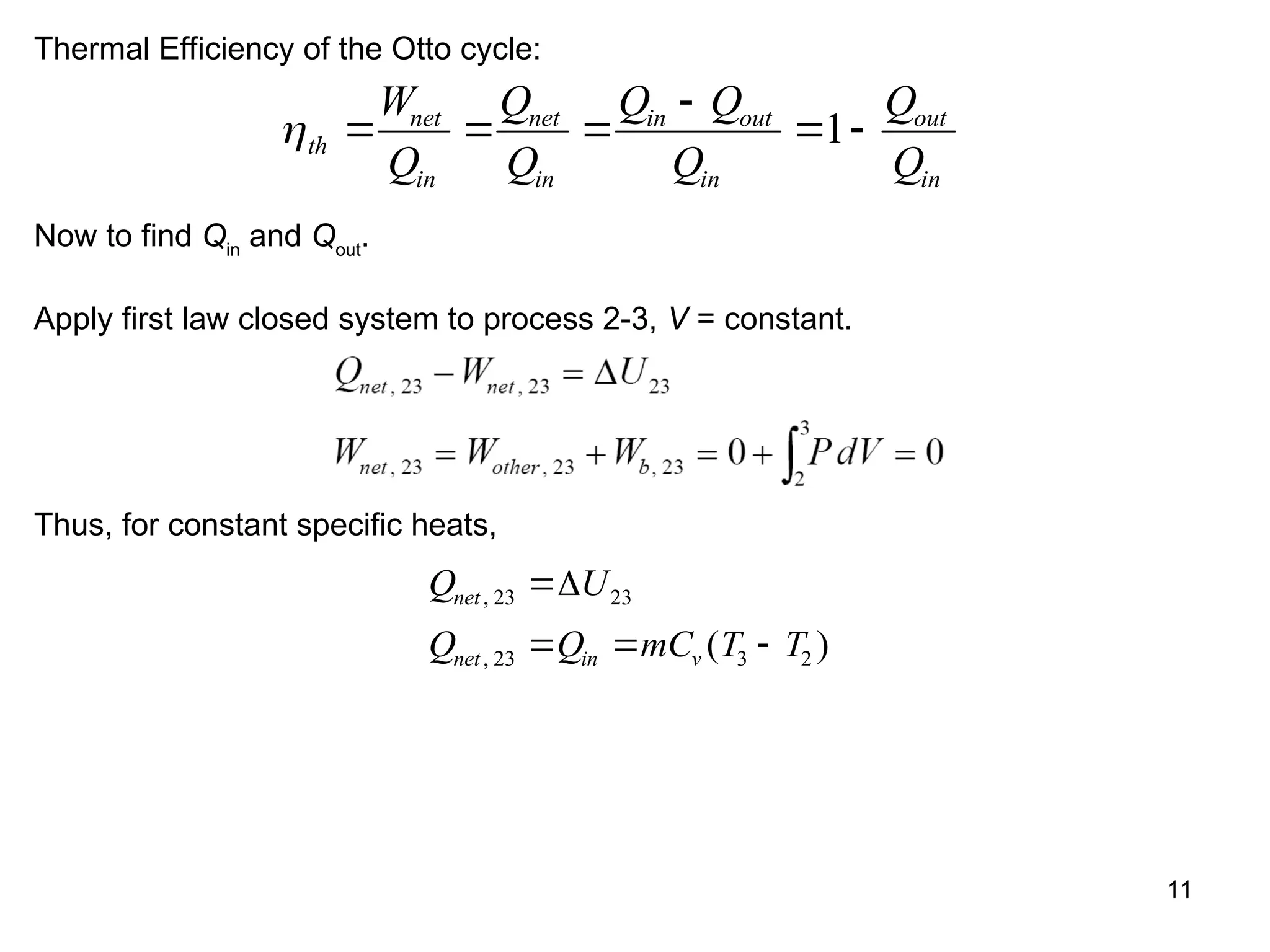
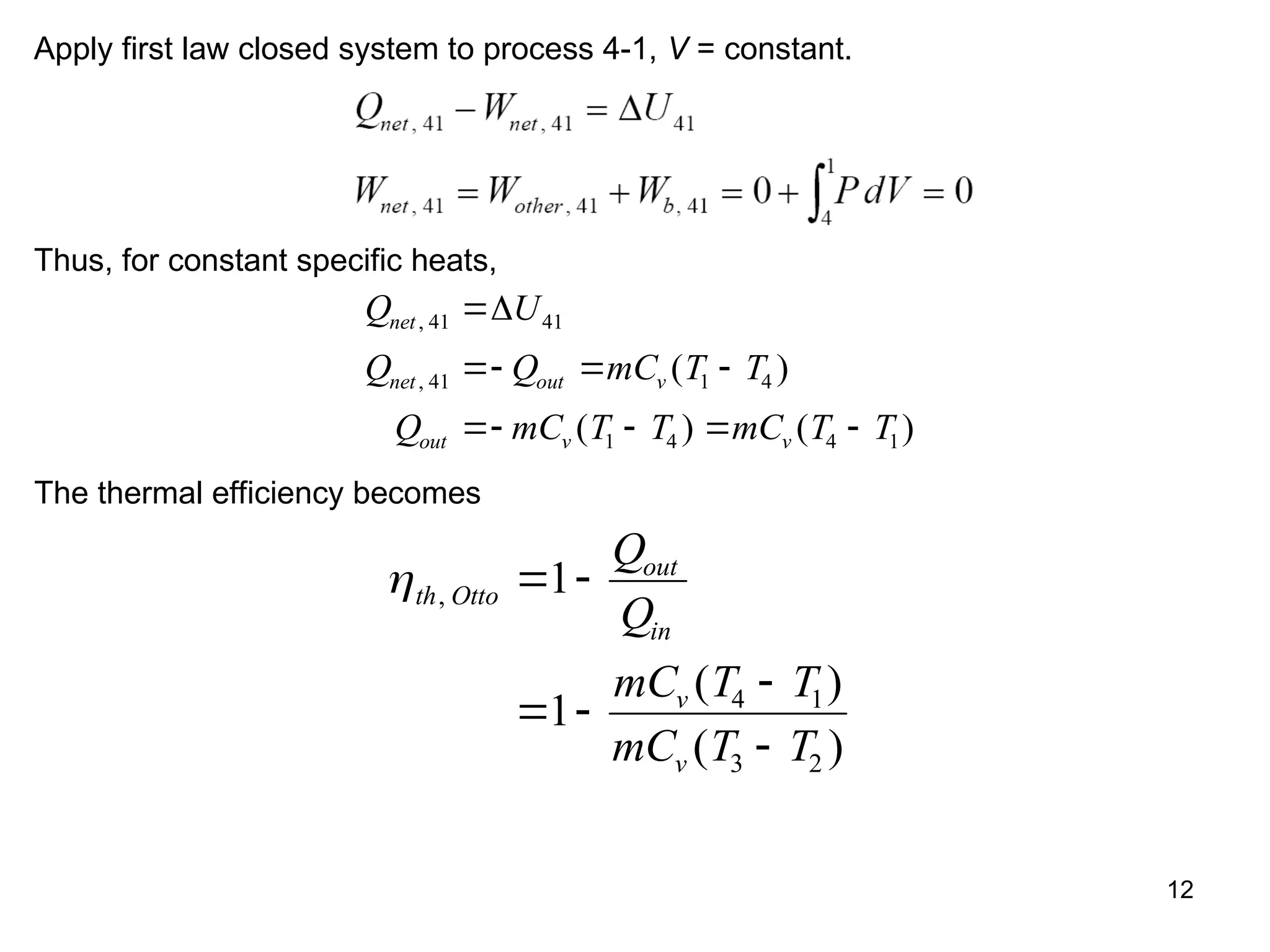
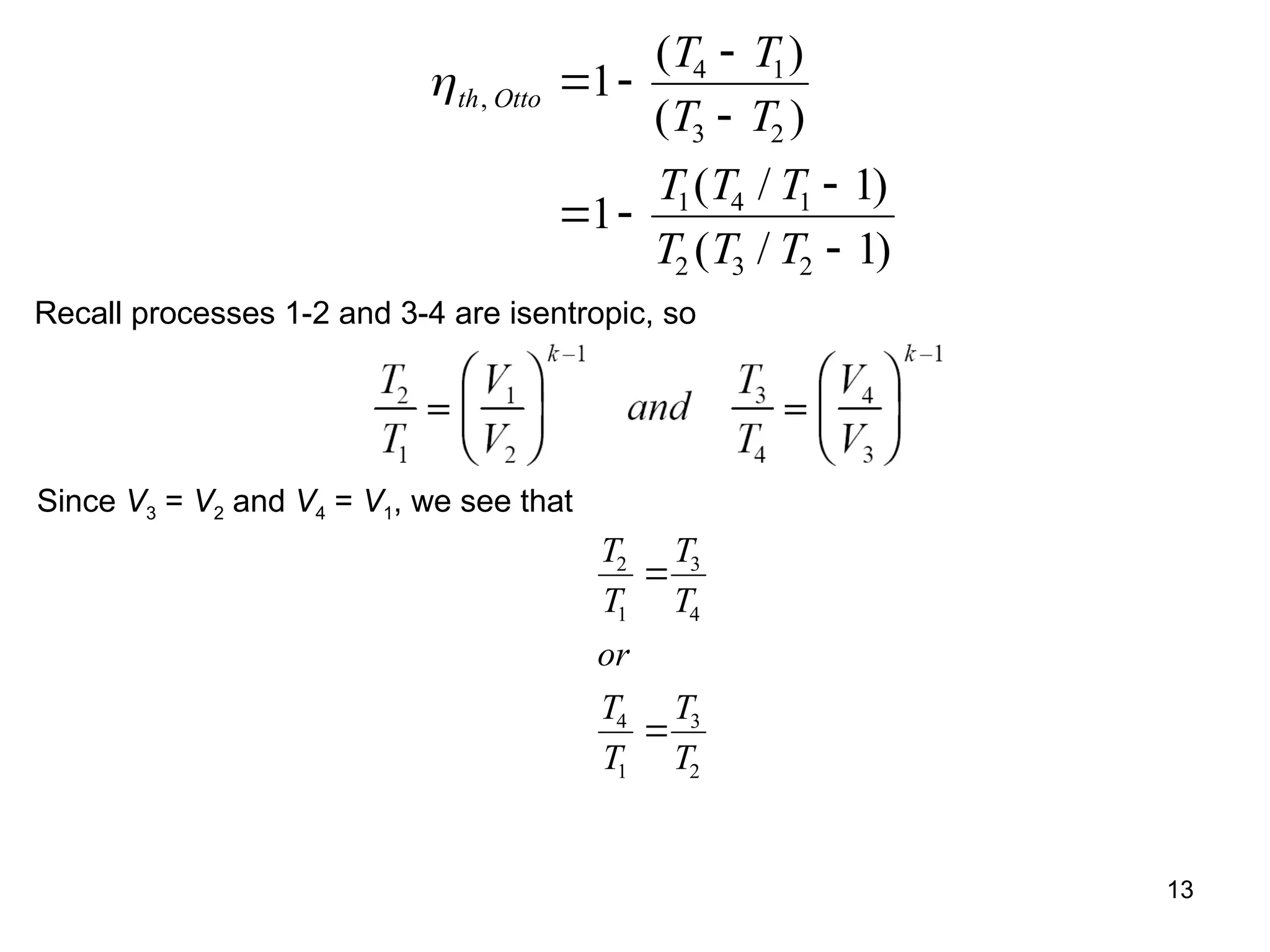
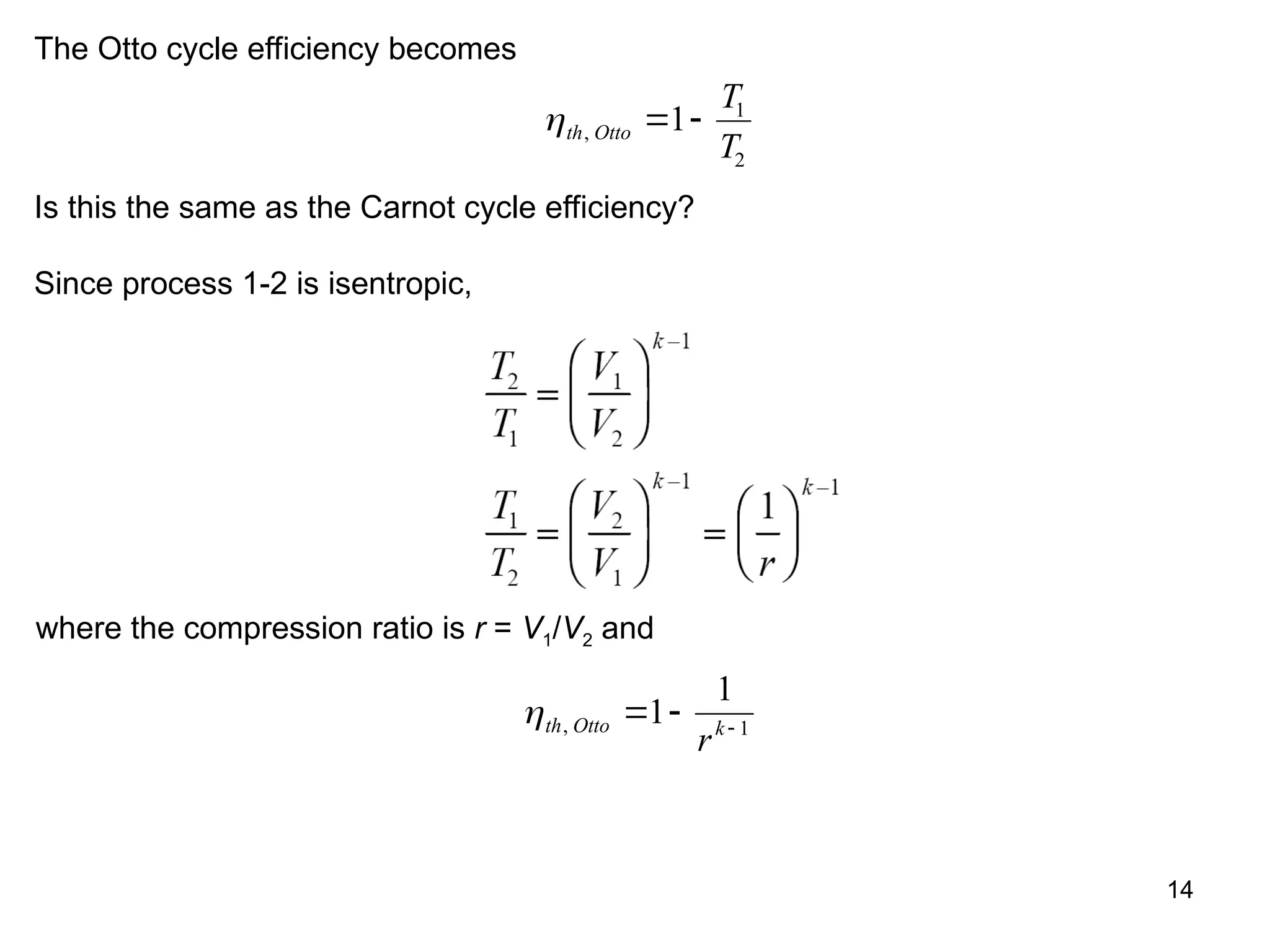
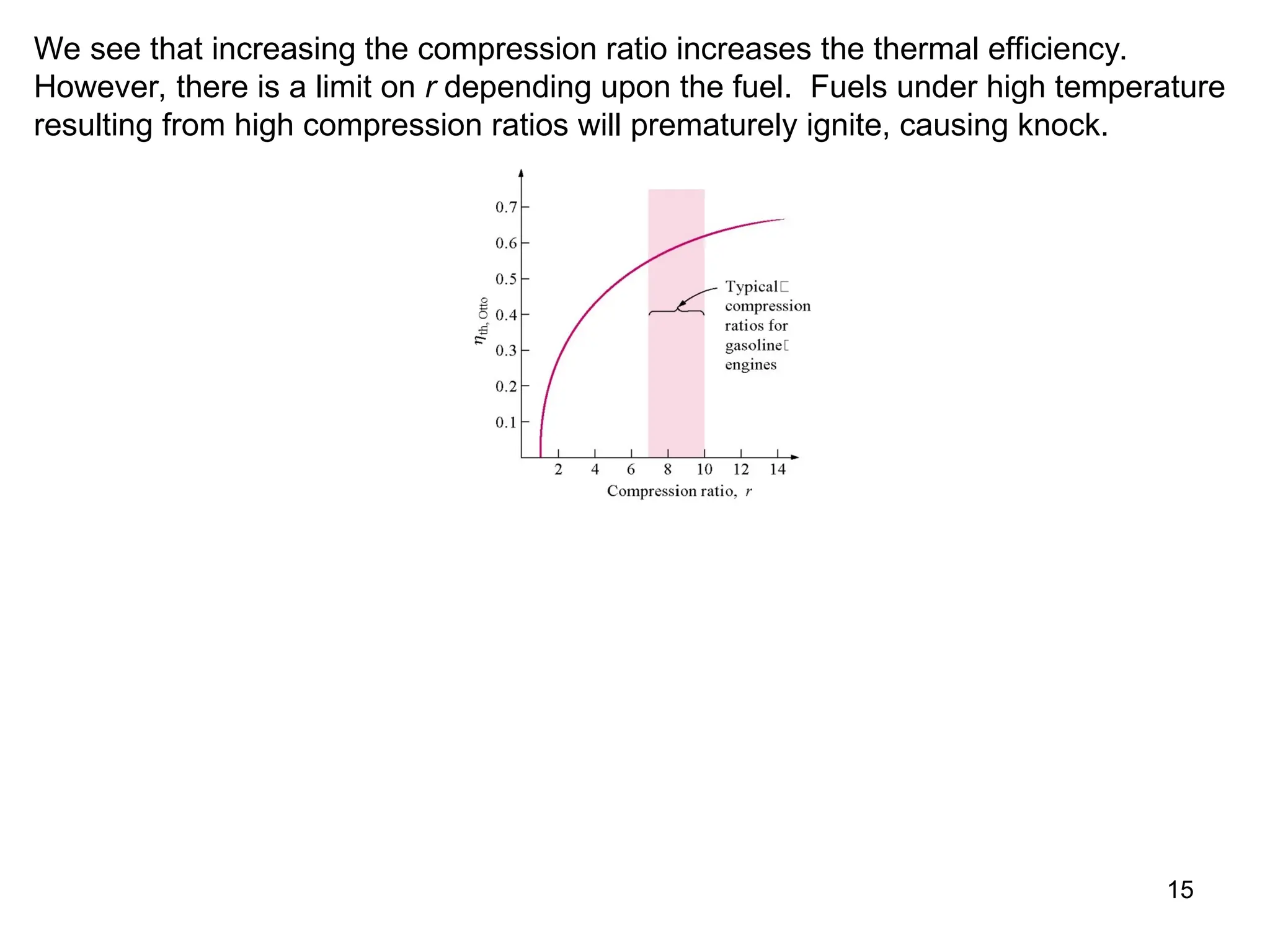
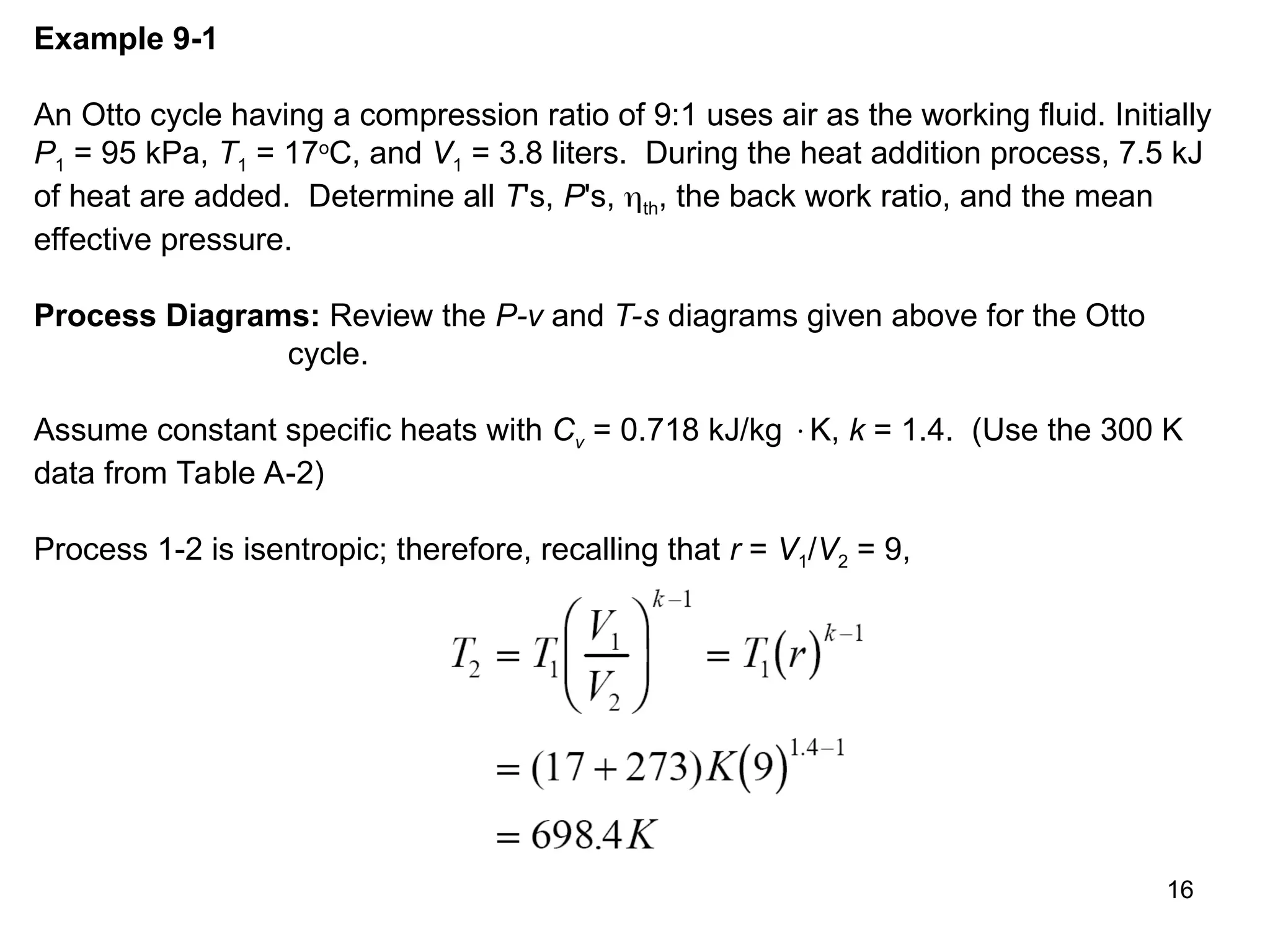
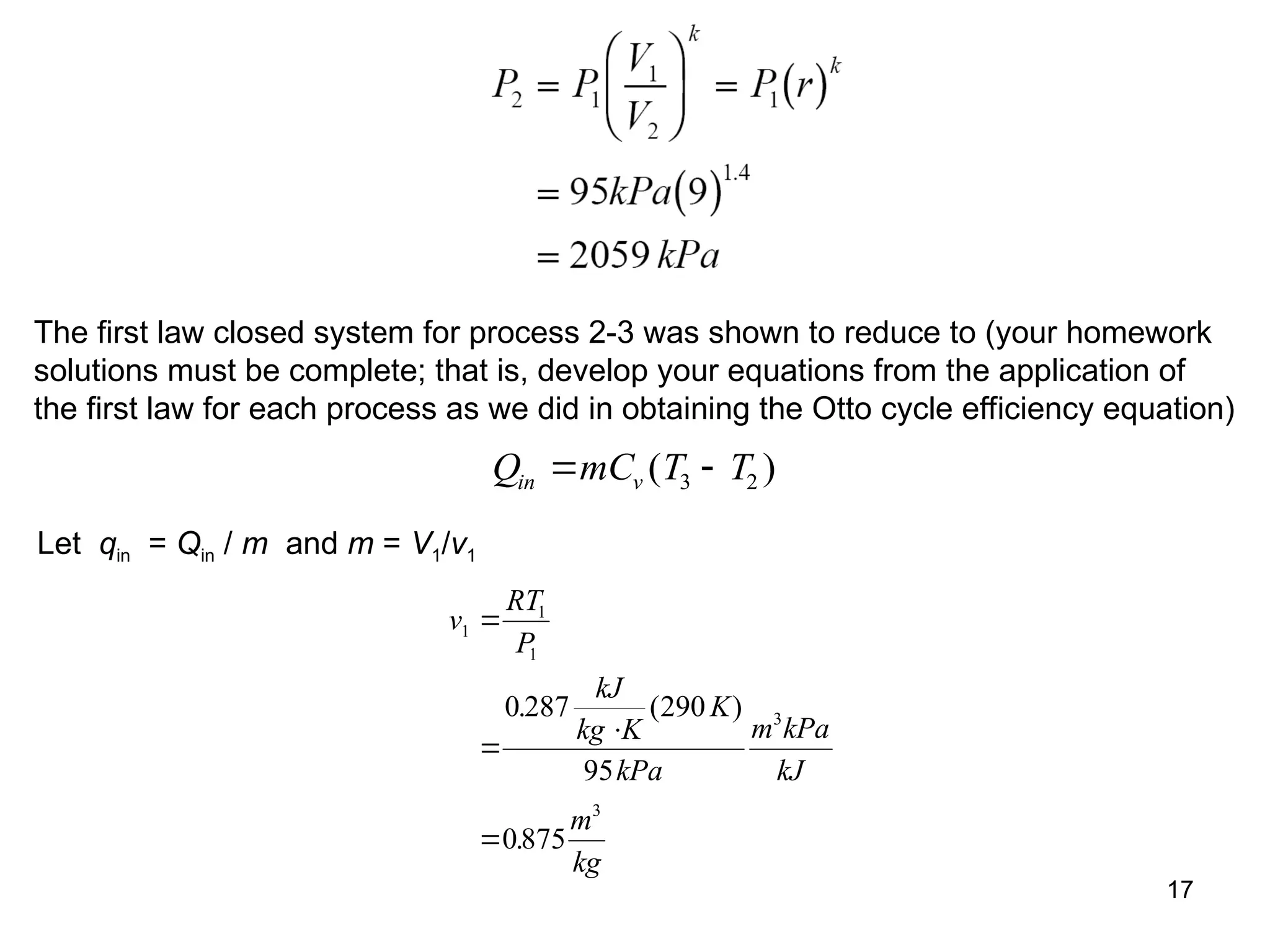
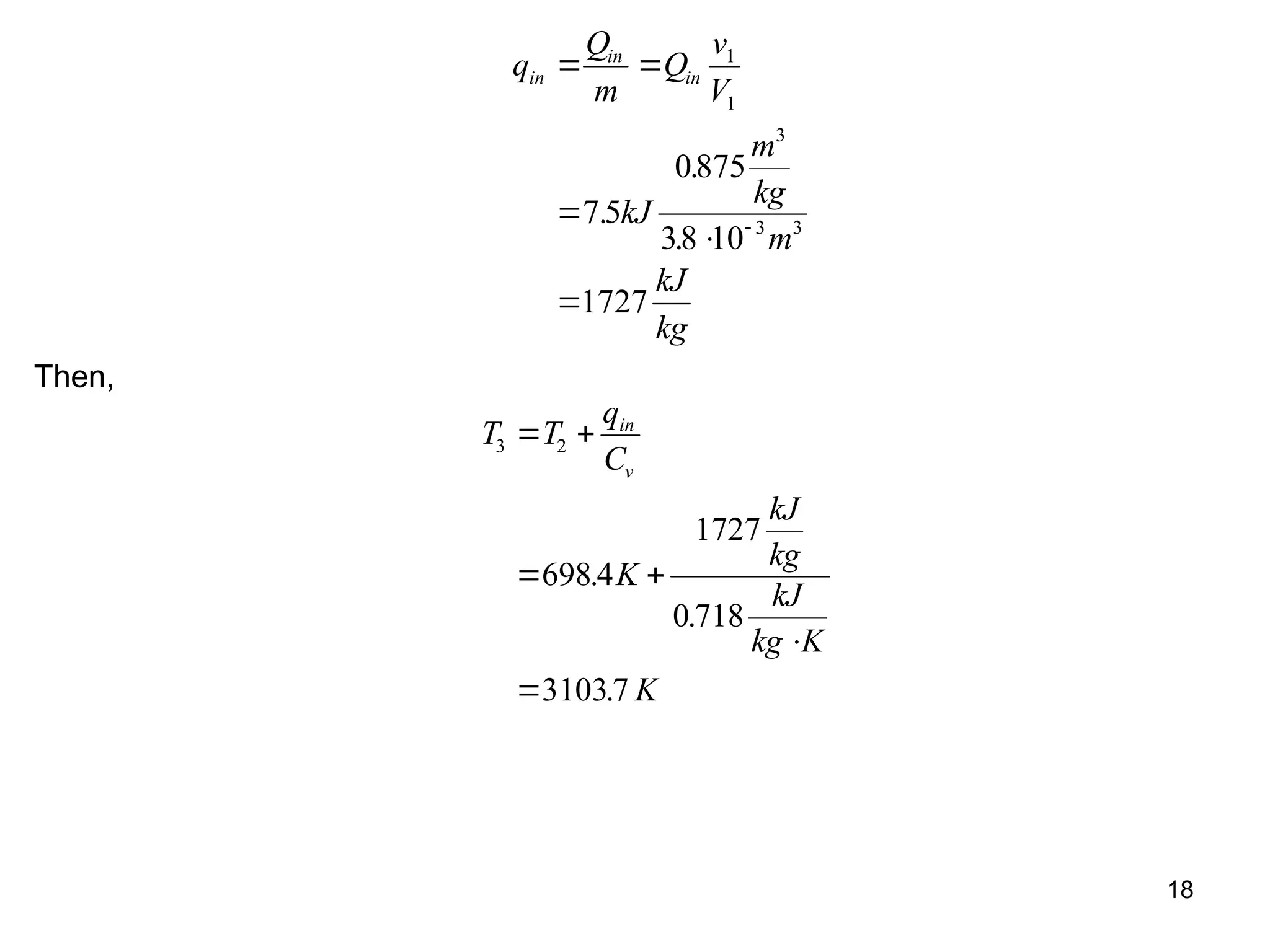
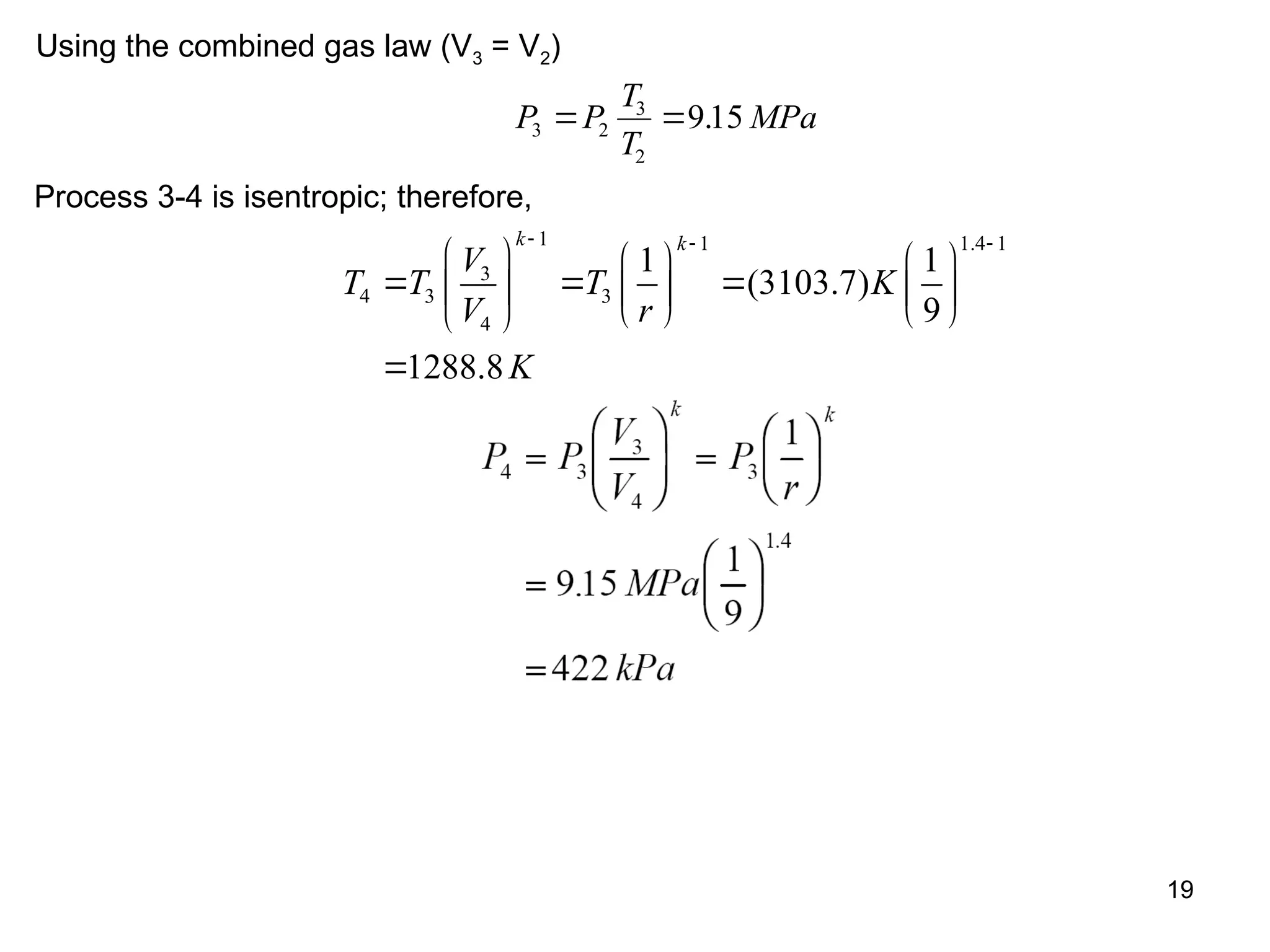
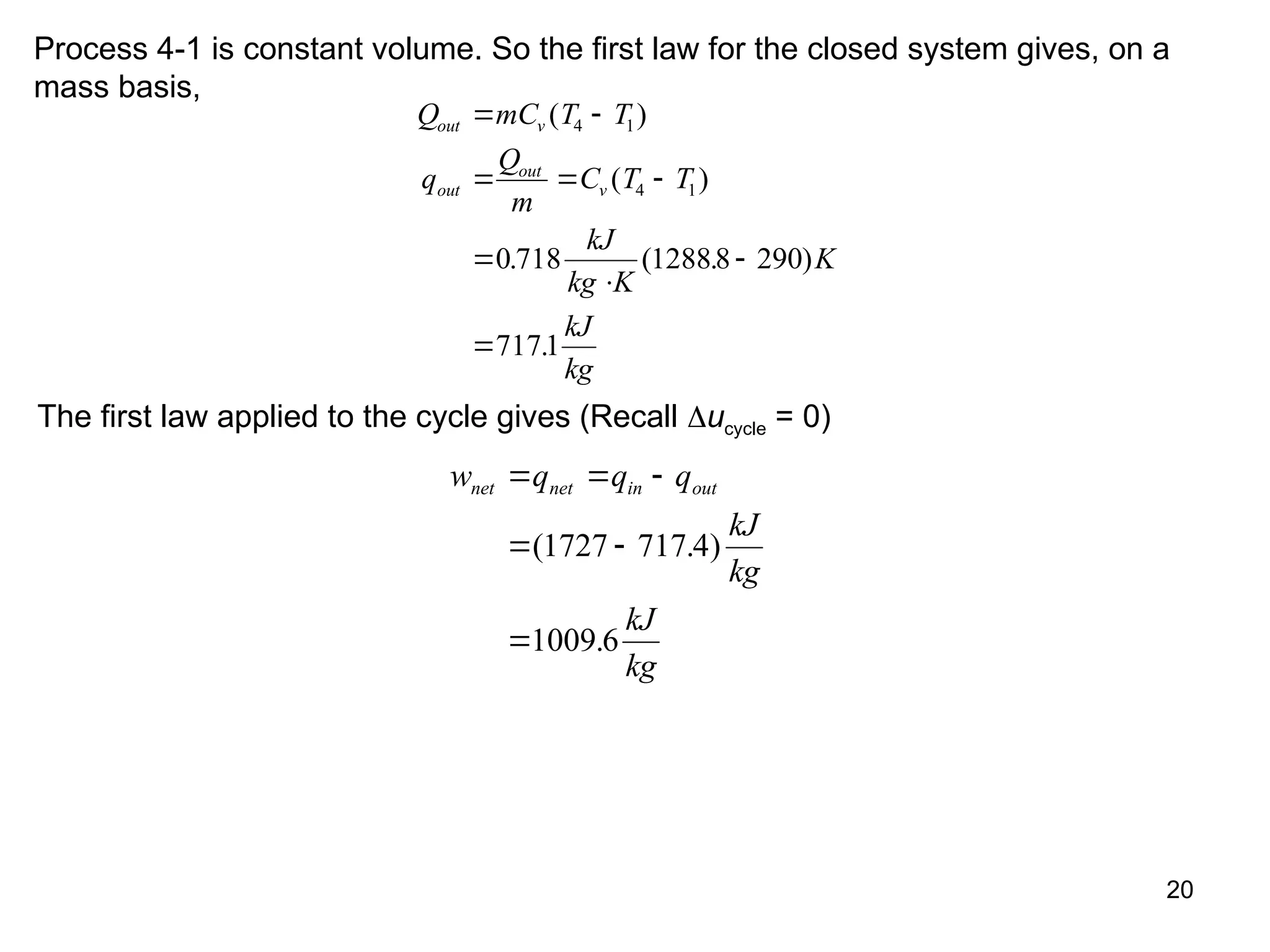
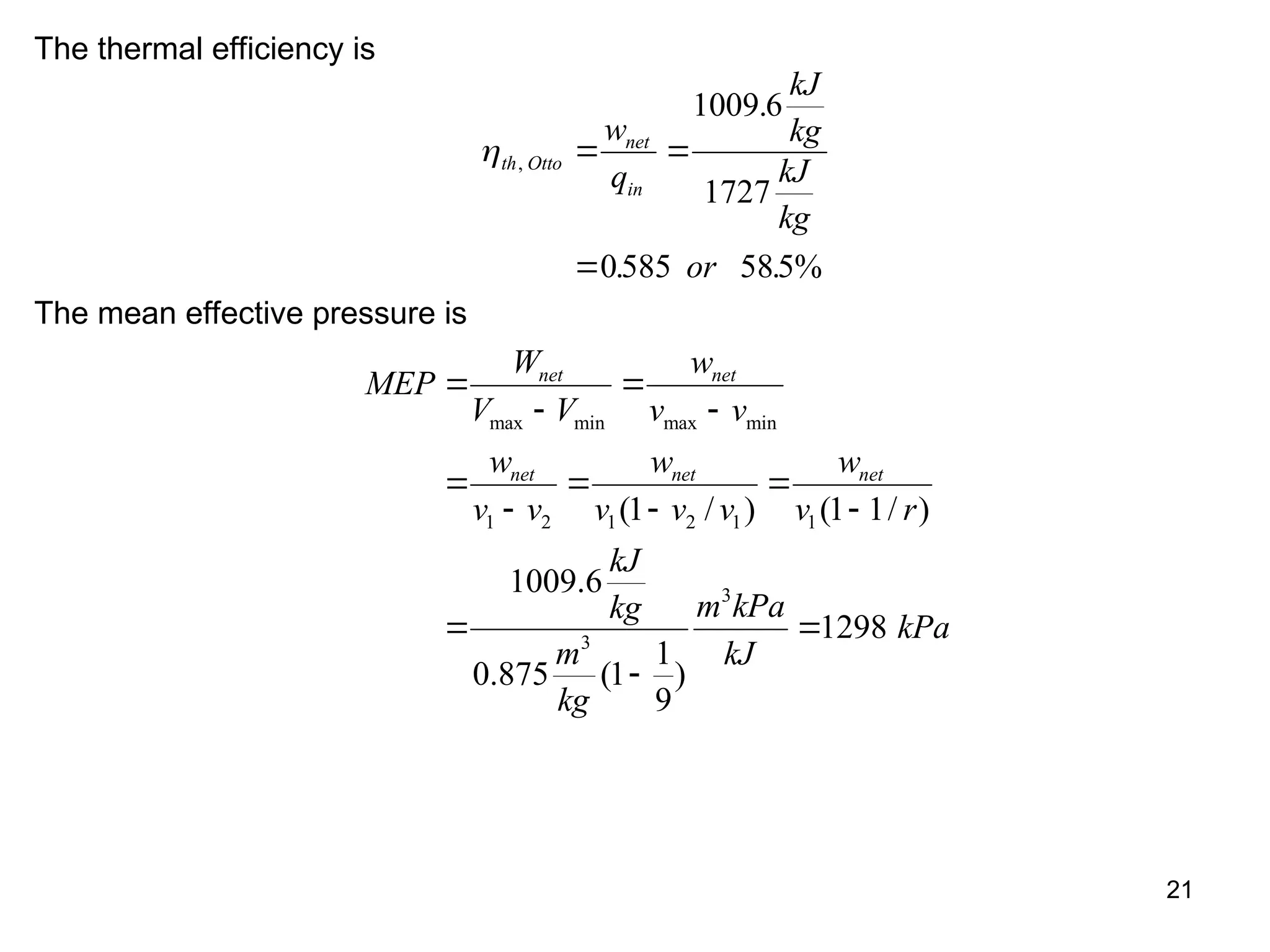
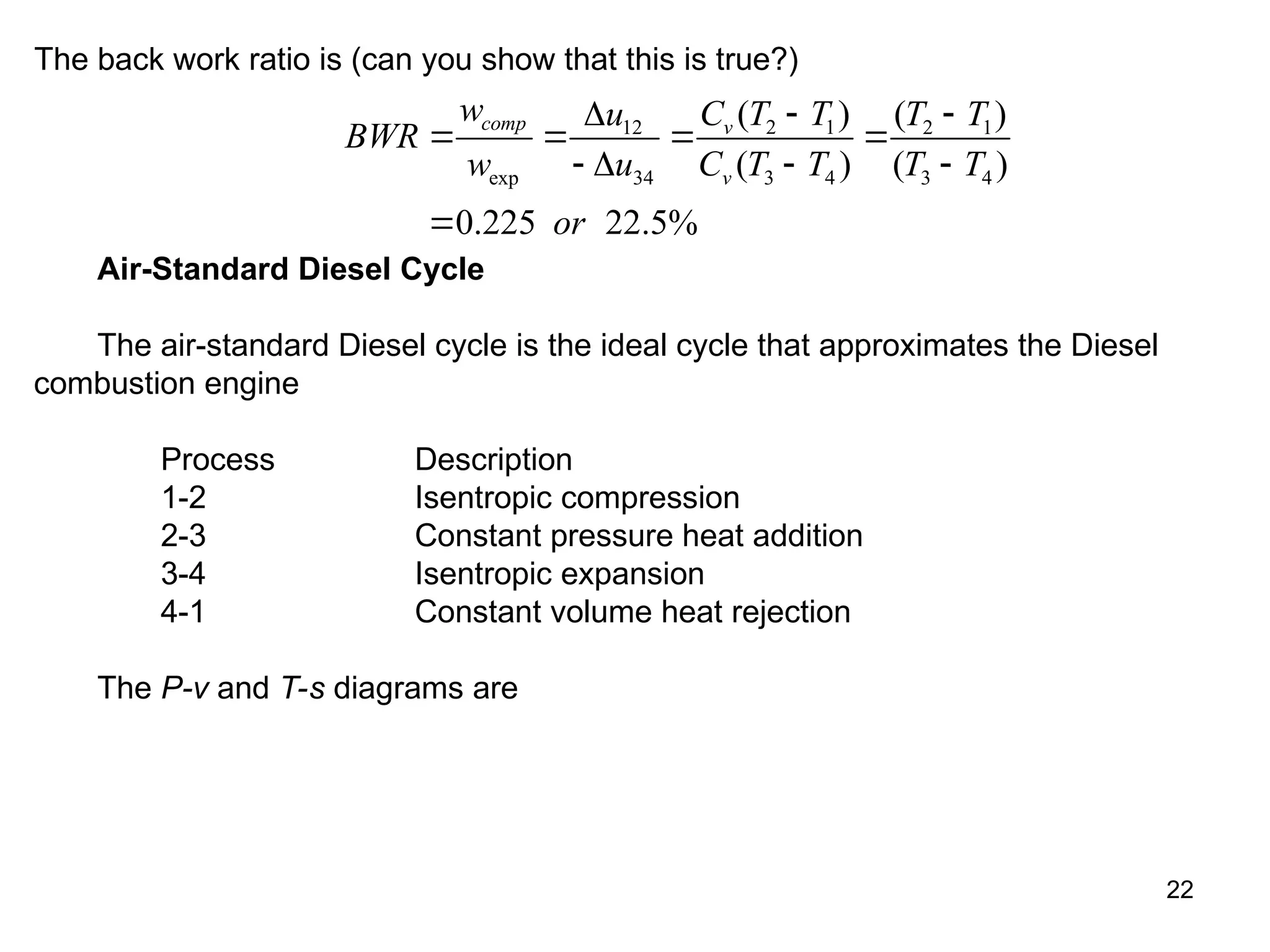
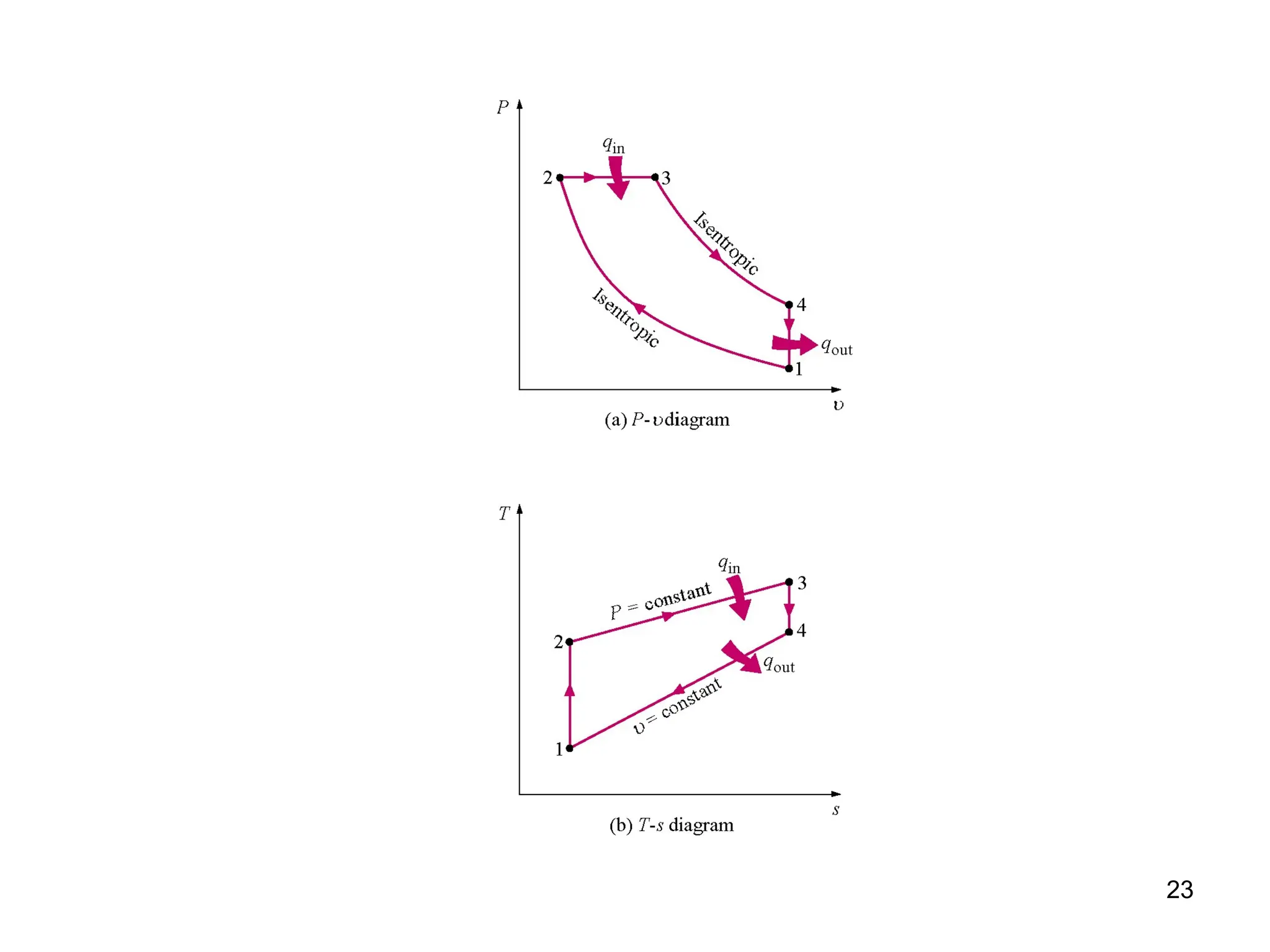
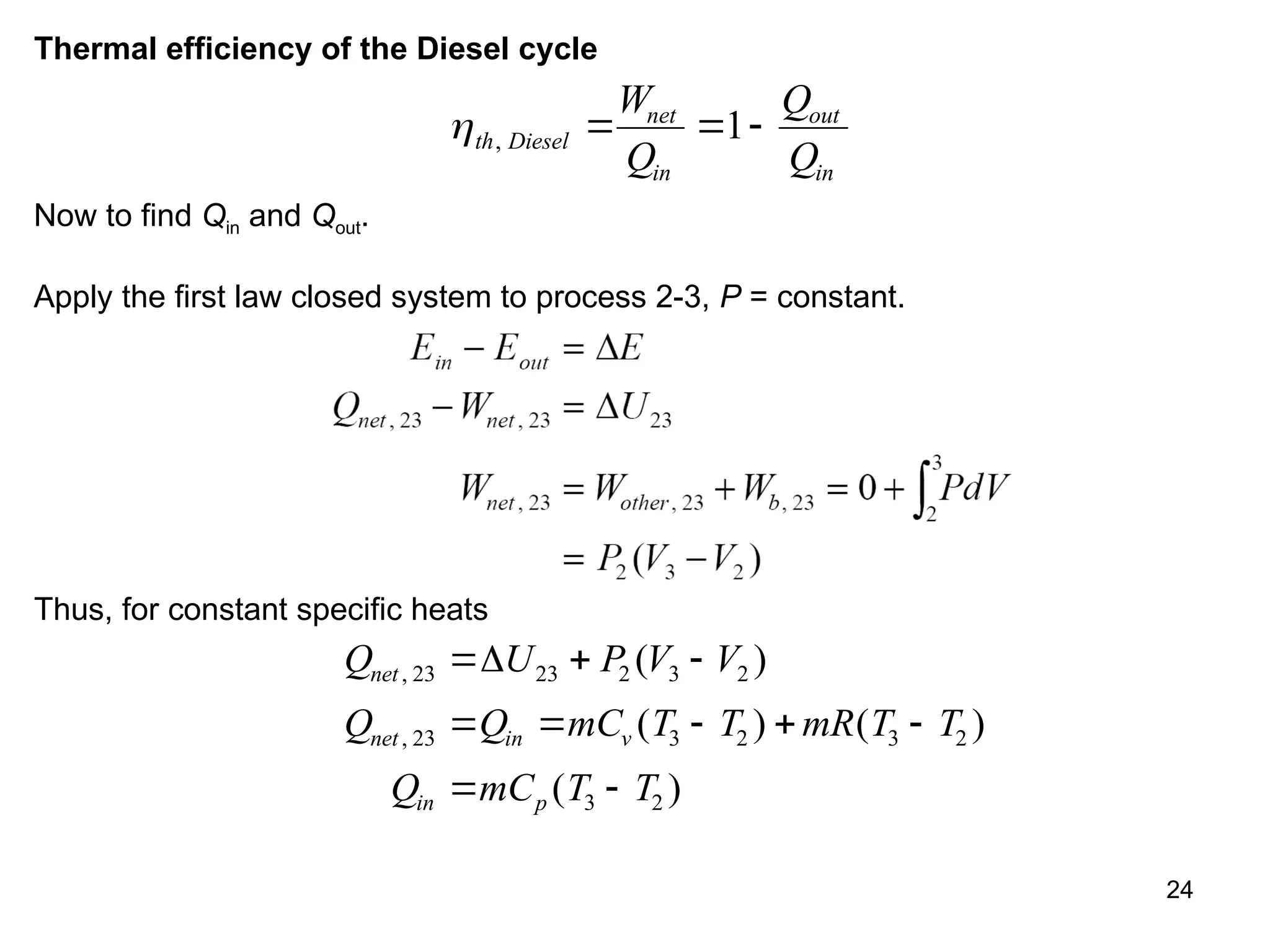
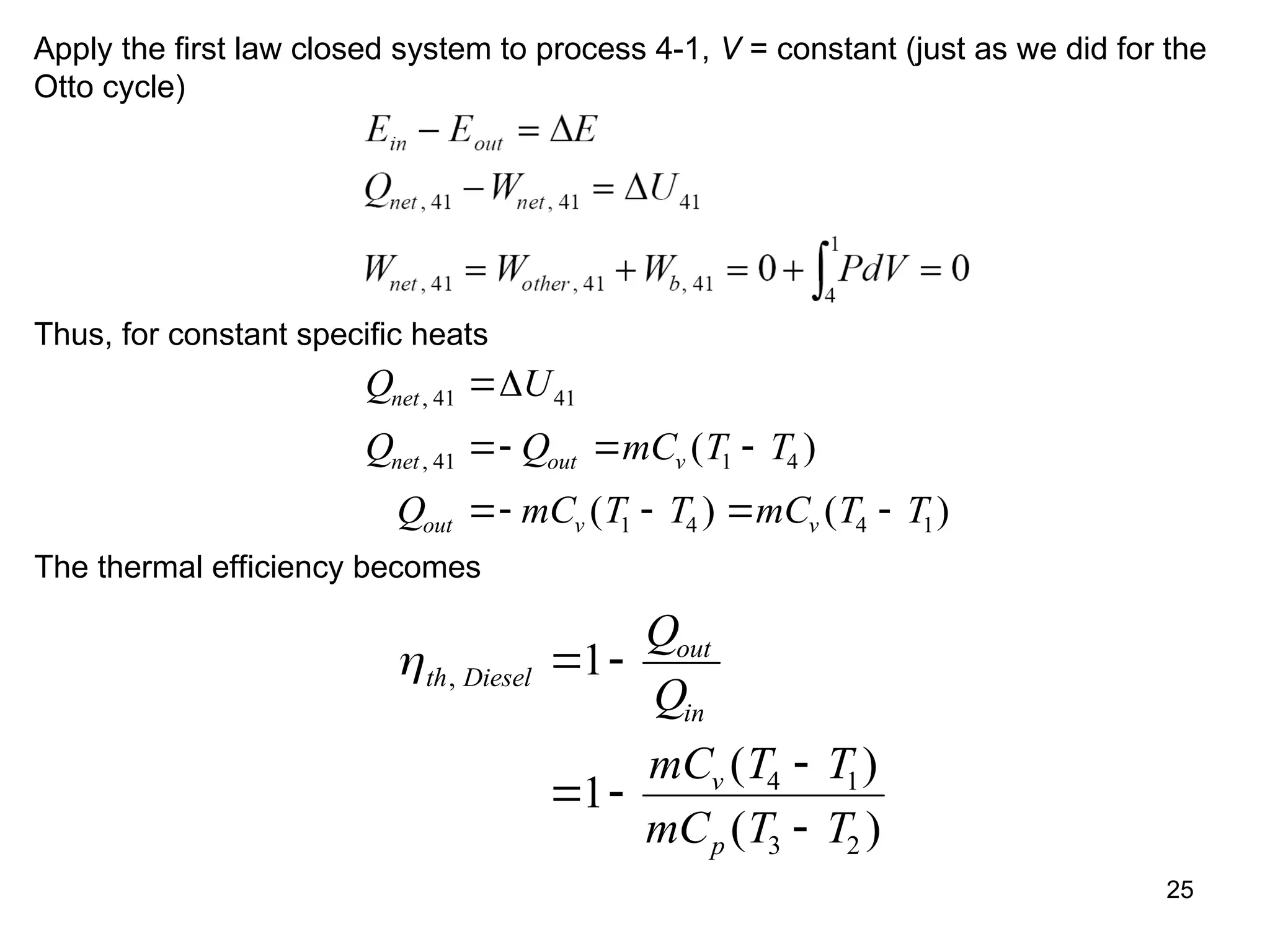
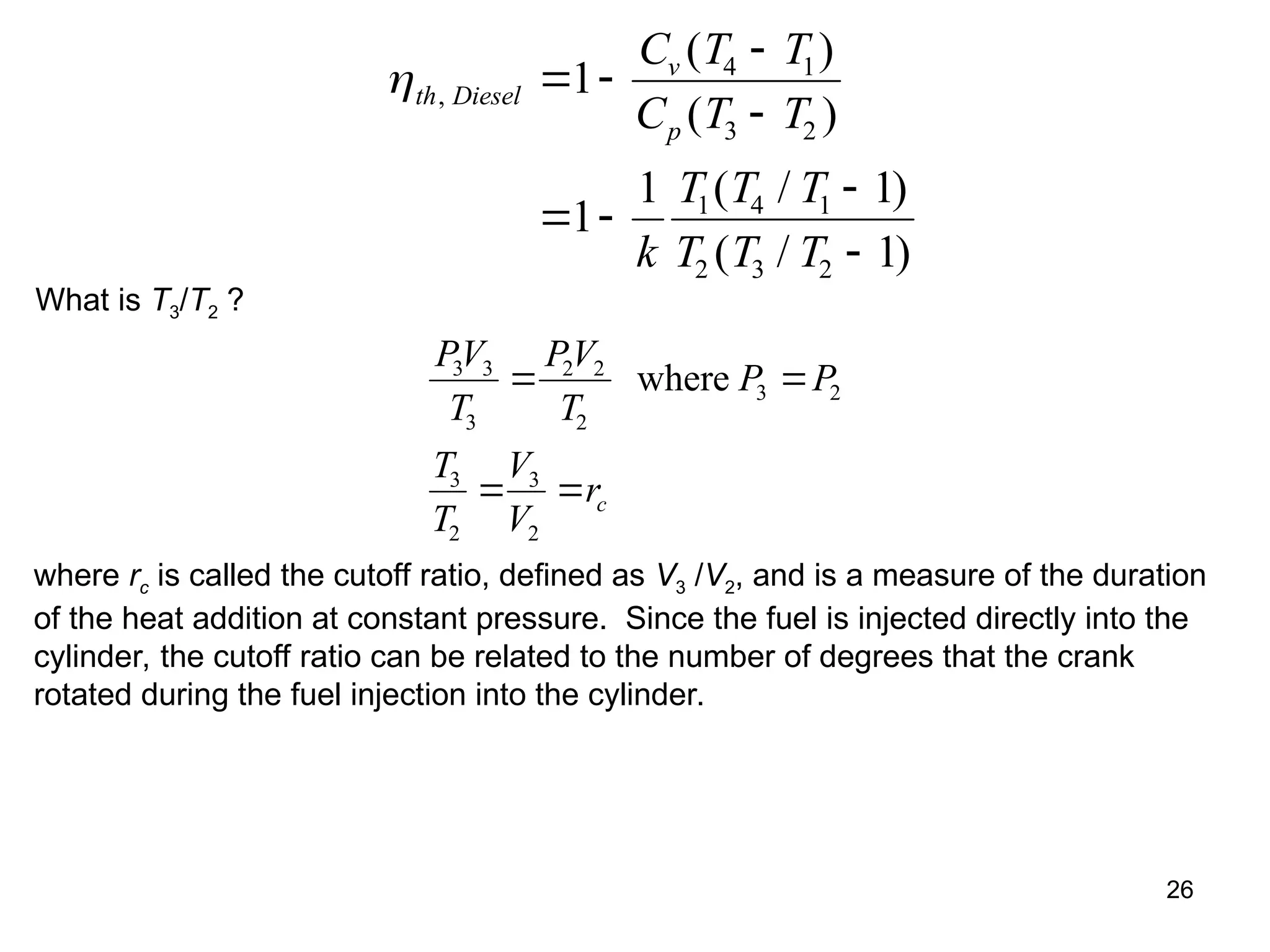
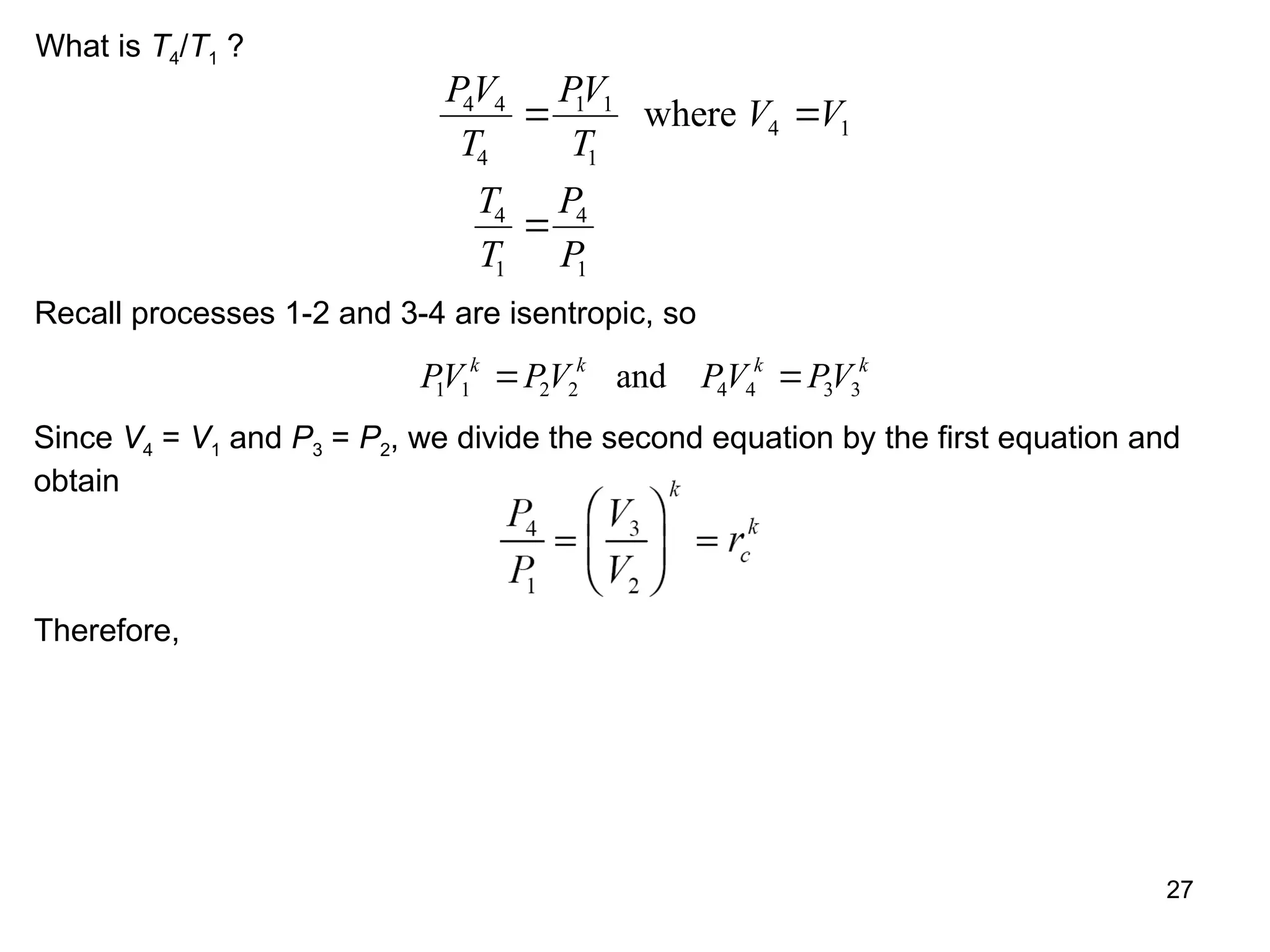
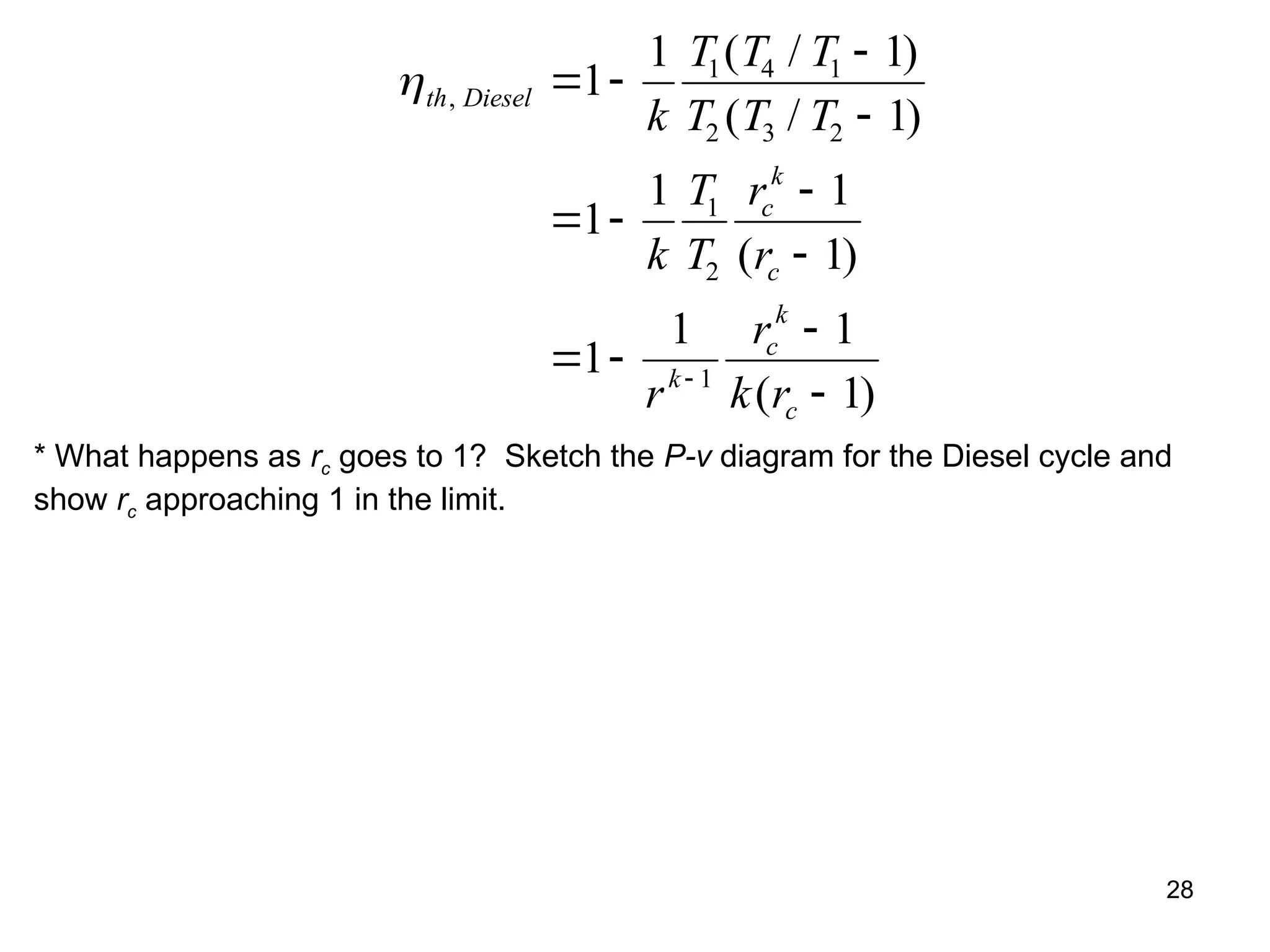
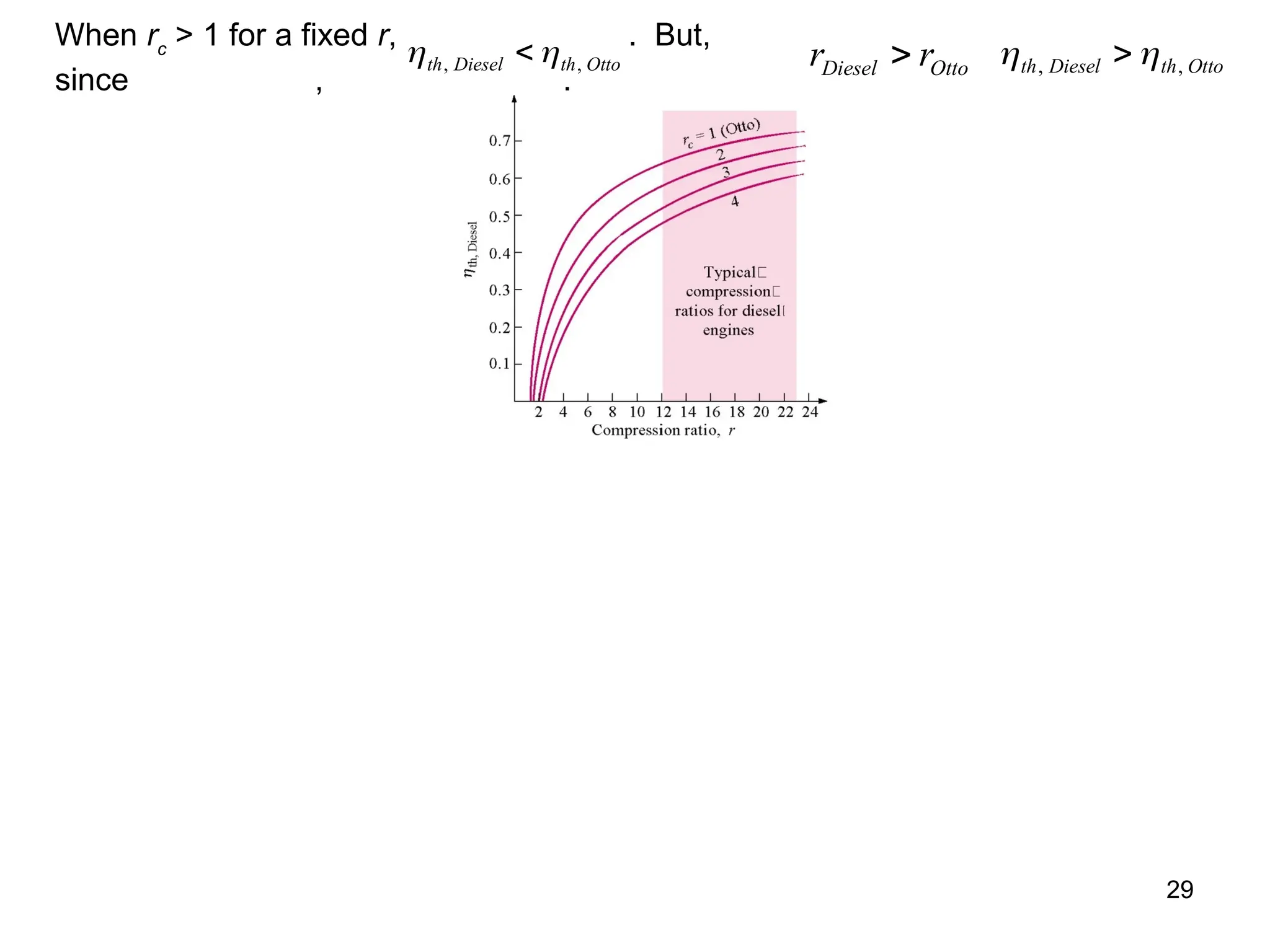
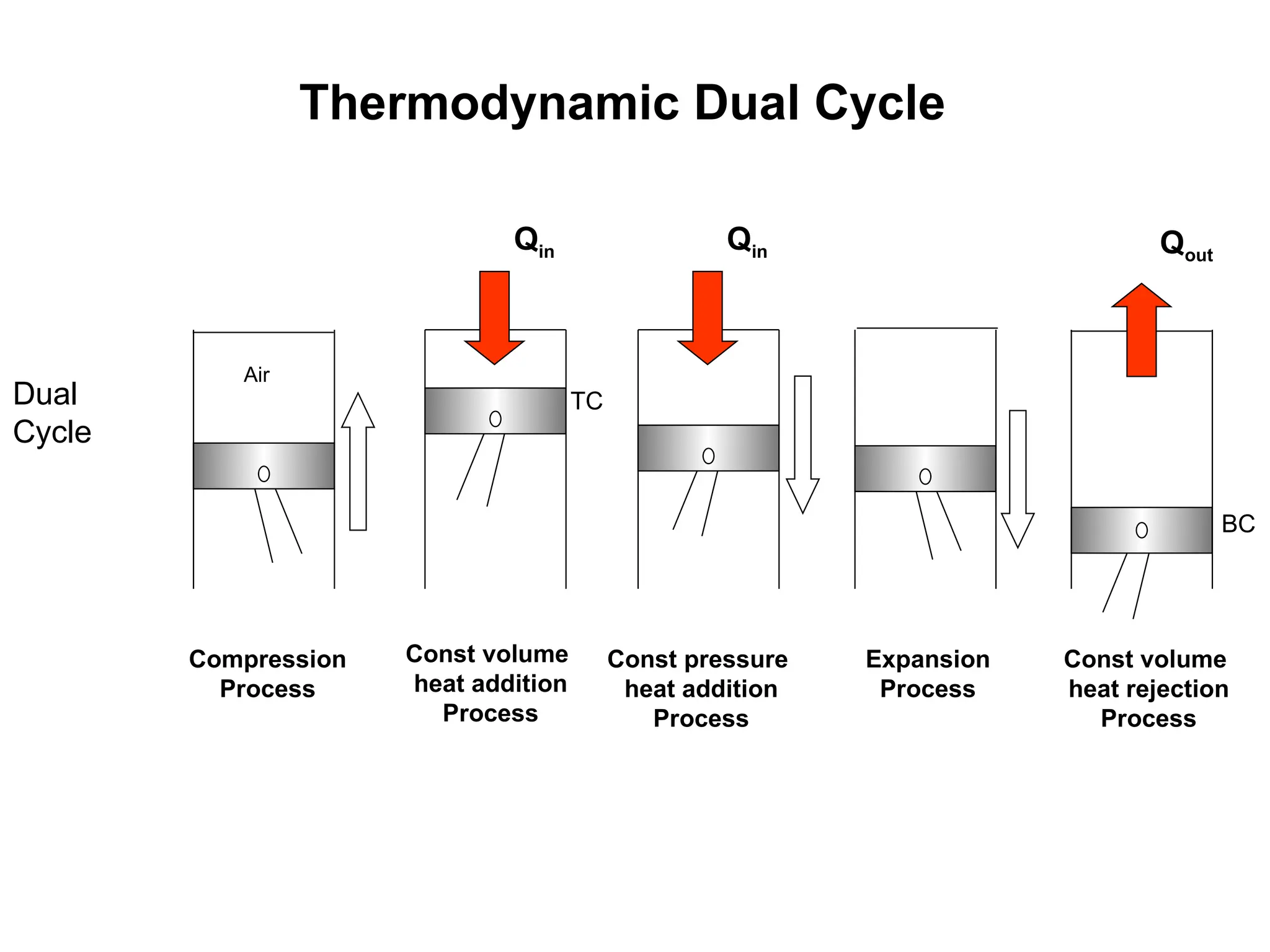
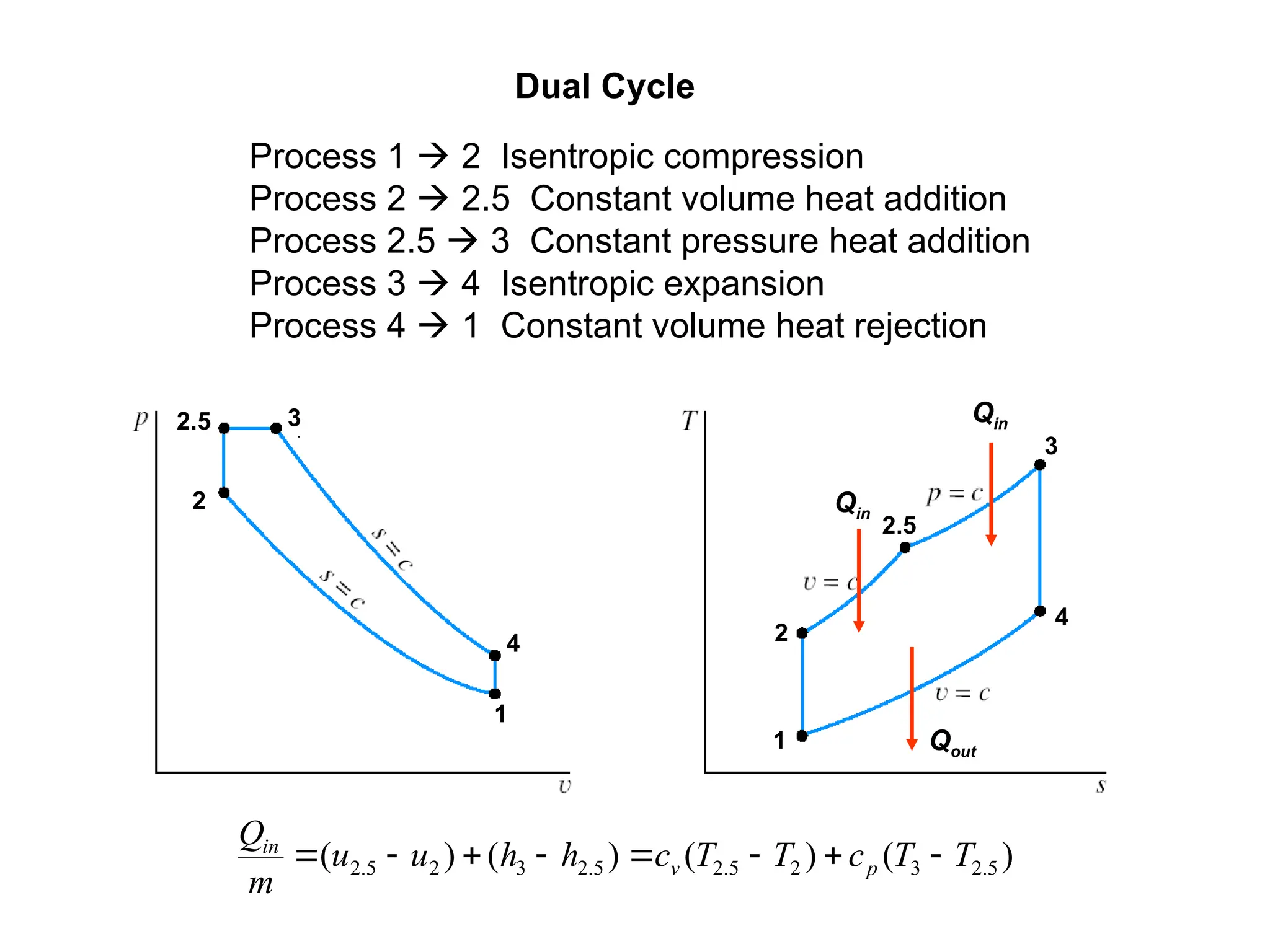
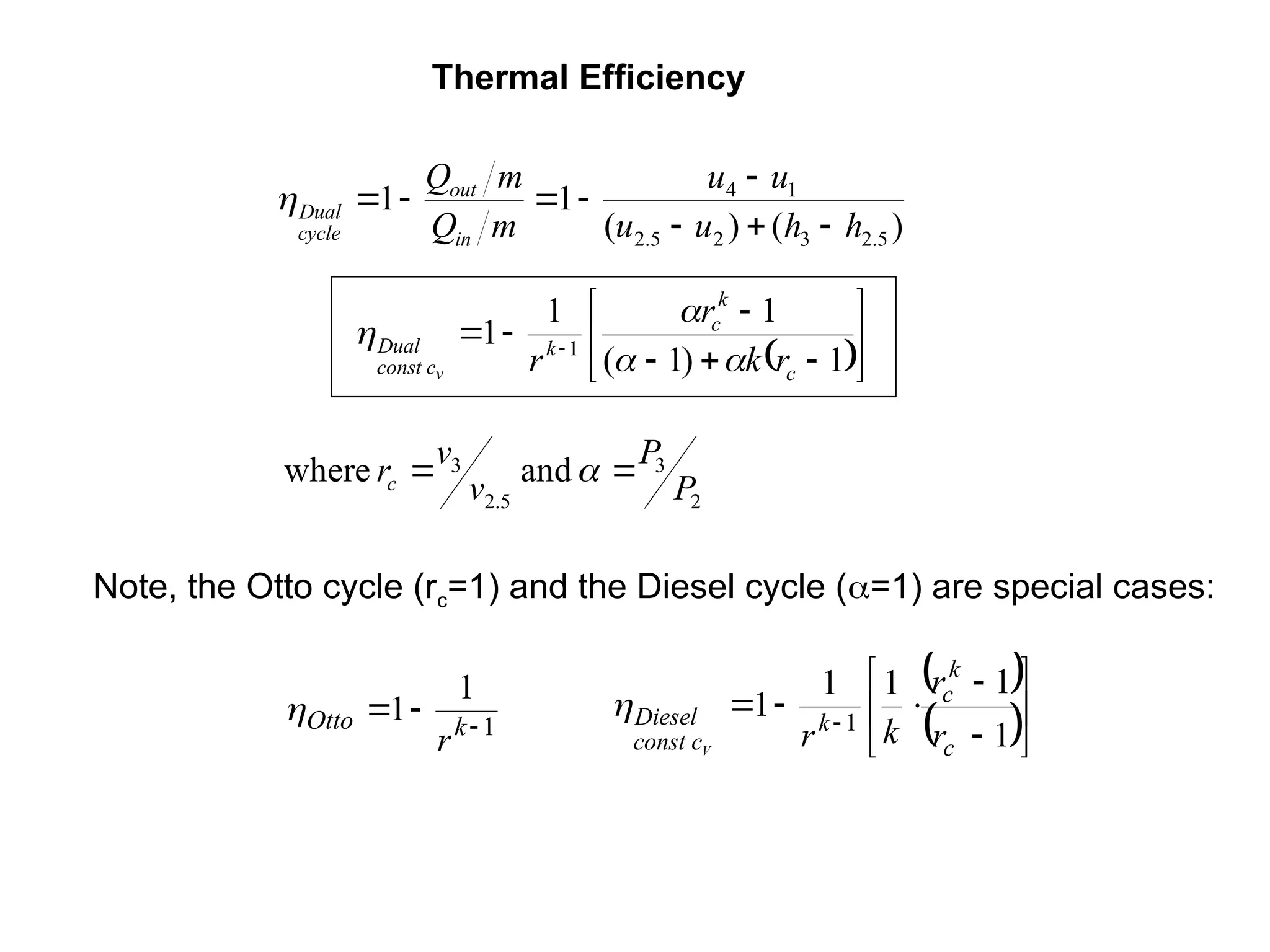
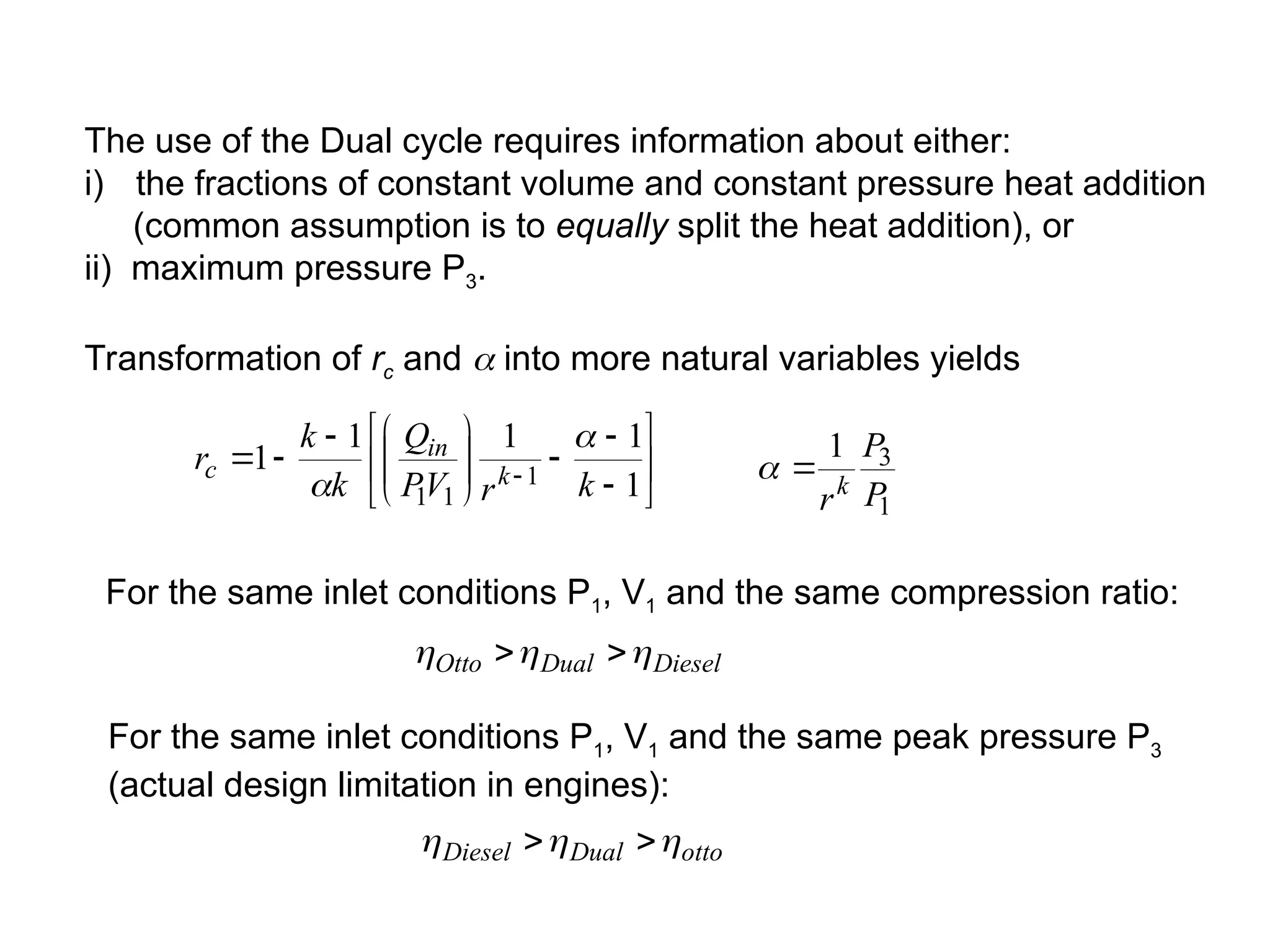
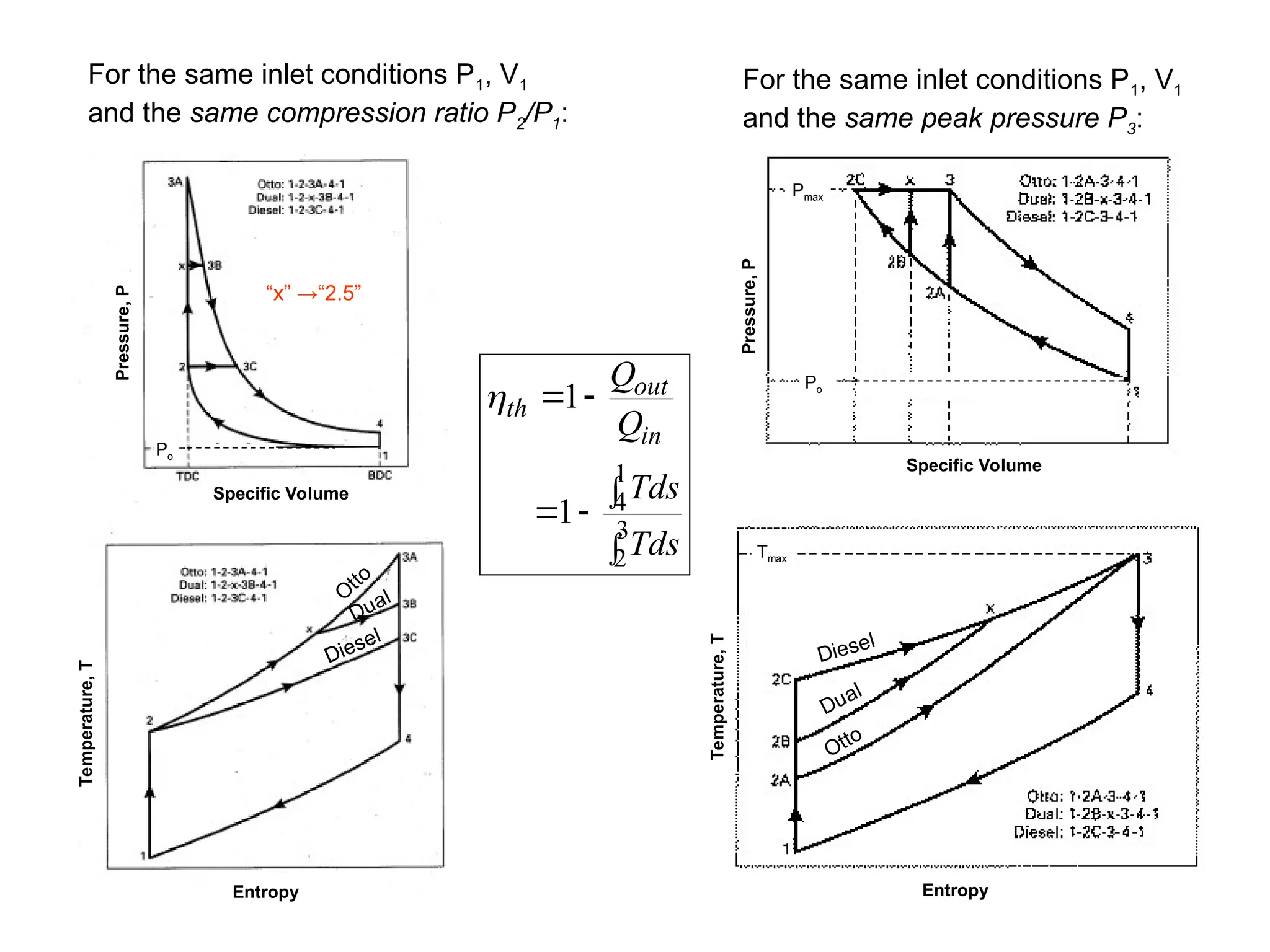
This document explores gas power cycles, focusing on ideal cycles like the Carnot and Otto cycles, detailing their processes and efficiency calculations. It emphasizes the assumptions made in modeling these cycles, such as treating air as an ideal gas and considering internally reversible processes. Additionally, it covers terms related to reciprocating engines and presents analysis on thermal efficiencies of both Otto and Diesel cycles.