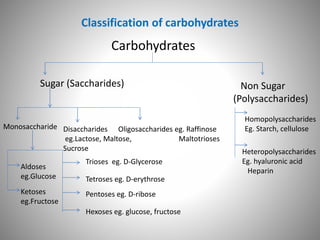
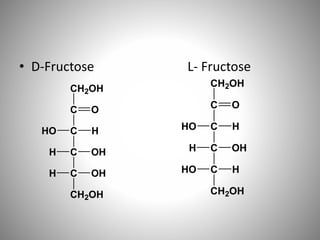
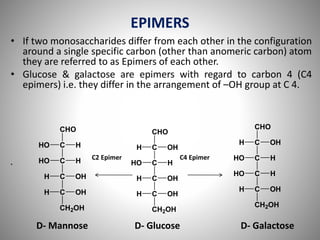
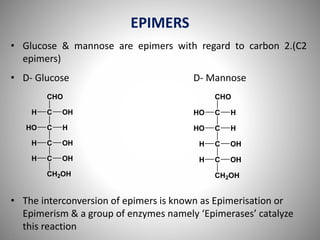
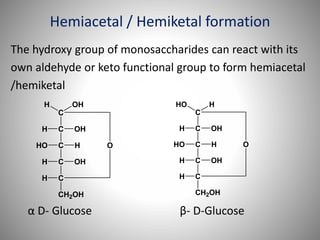
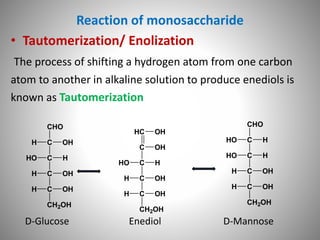
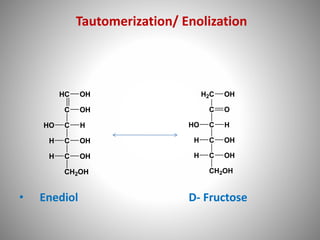
Carbohydrates are defined as polyhydroxy aldehydes or ketones, classified into sugars (monosaccharides, disaccharides, oligosaccharides) and non-sugars (polysaccharides). They serve various functions including energy supply, structural components in cell membranes, and storage forms of energy. The document also details the biochemical reactions of carbohydrates, such as mutarotation, epimerization, and oxidation, along with their significance in tests for reducing sugars.