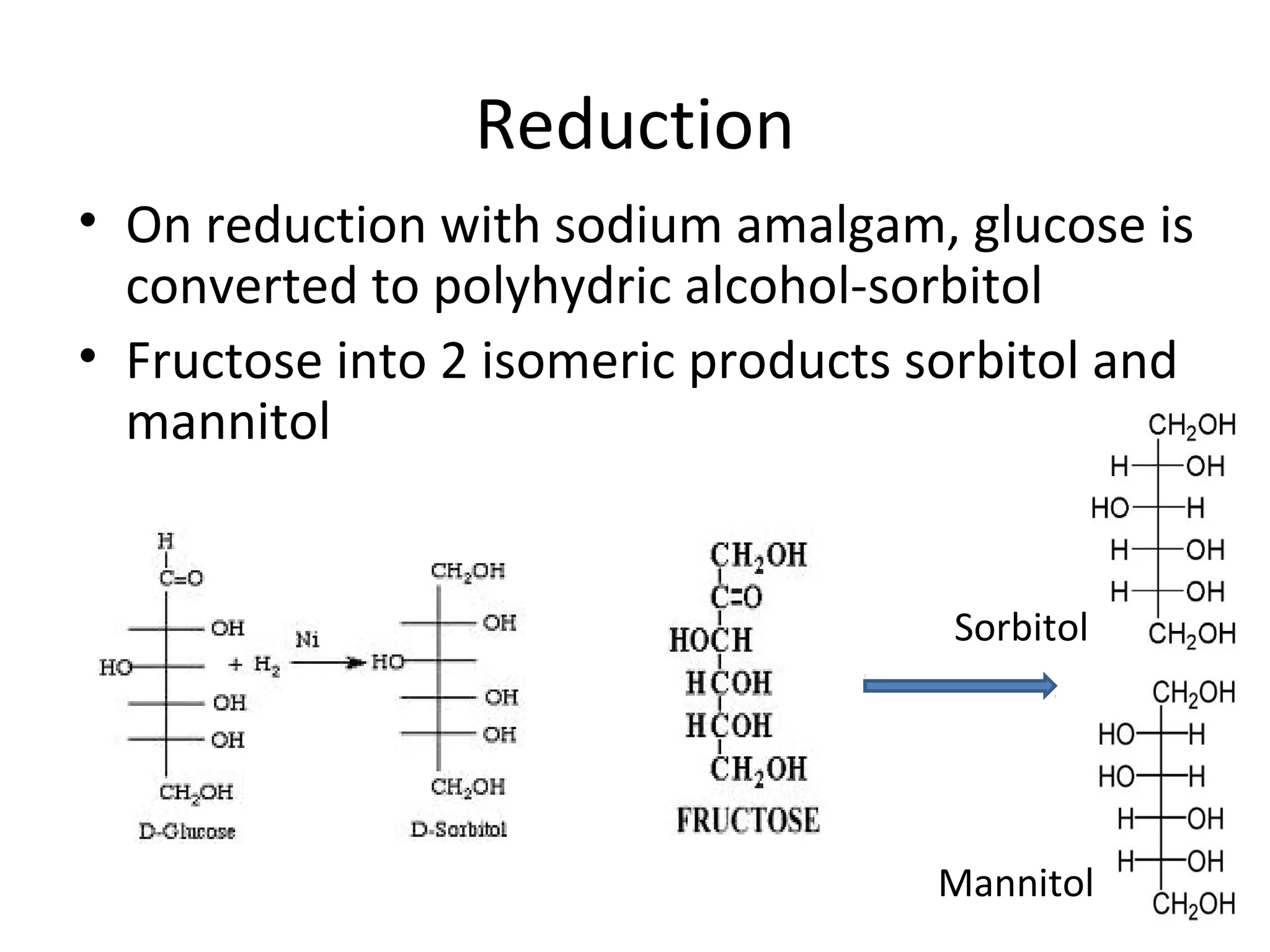
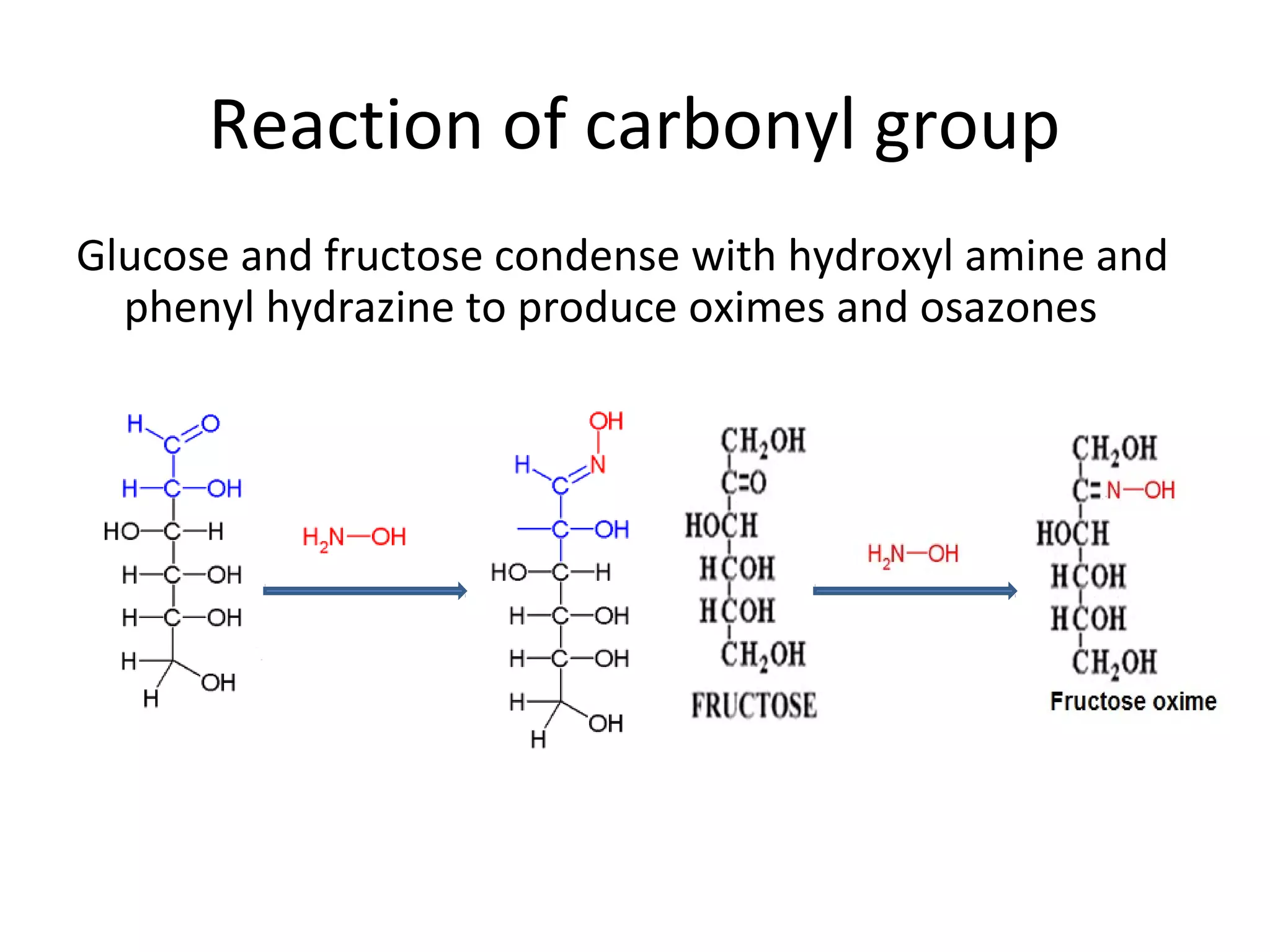
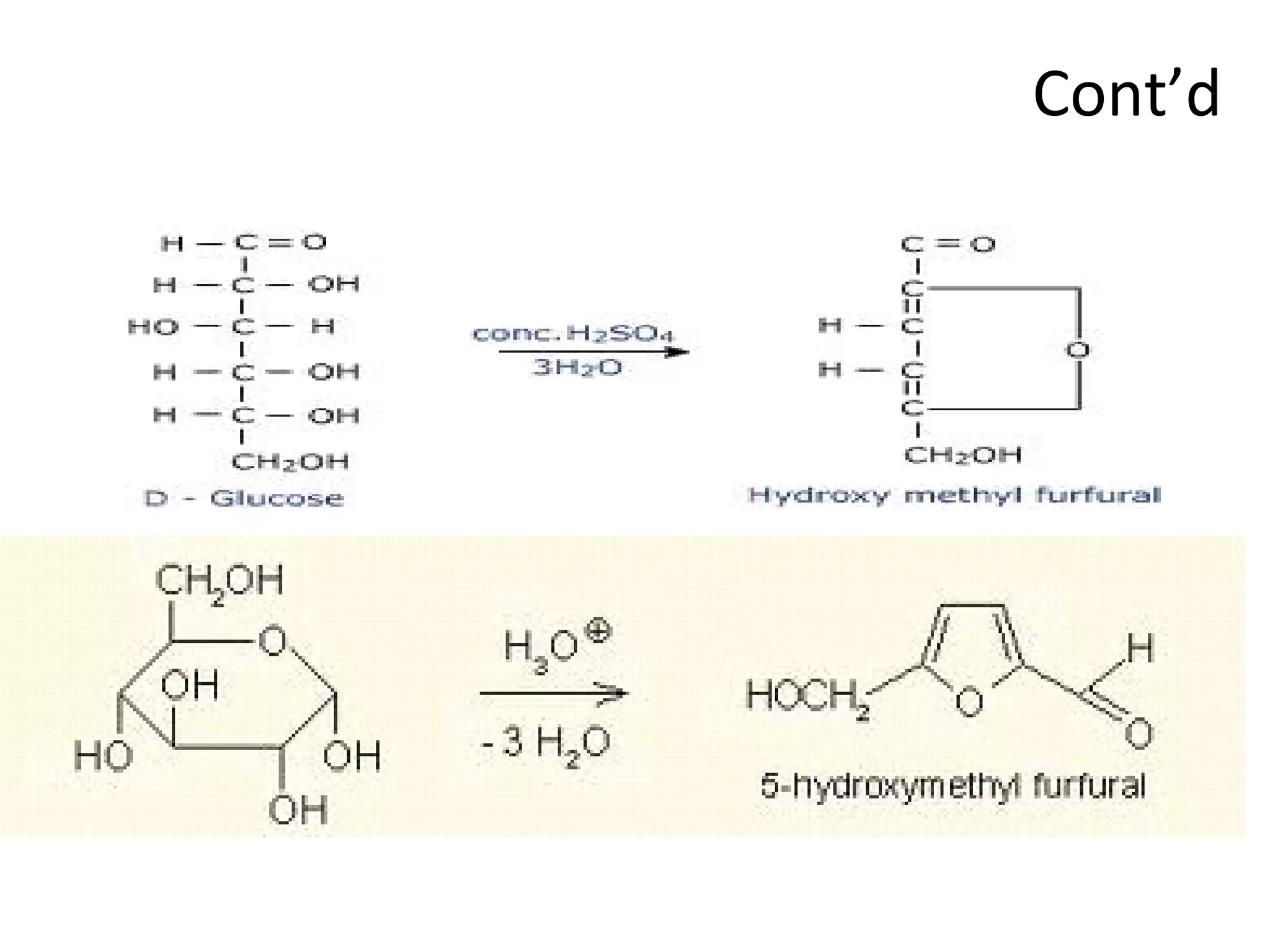
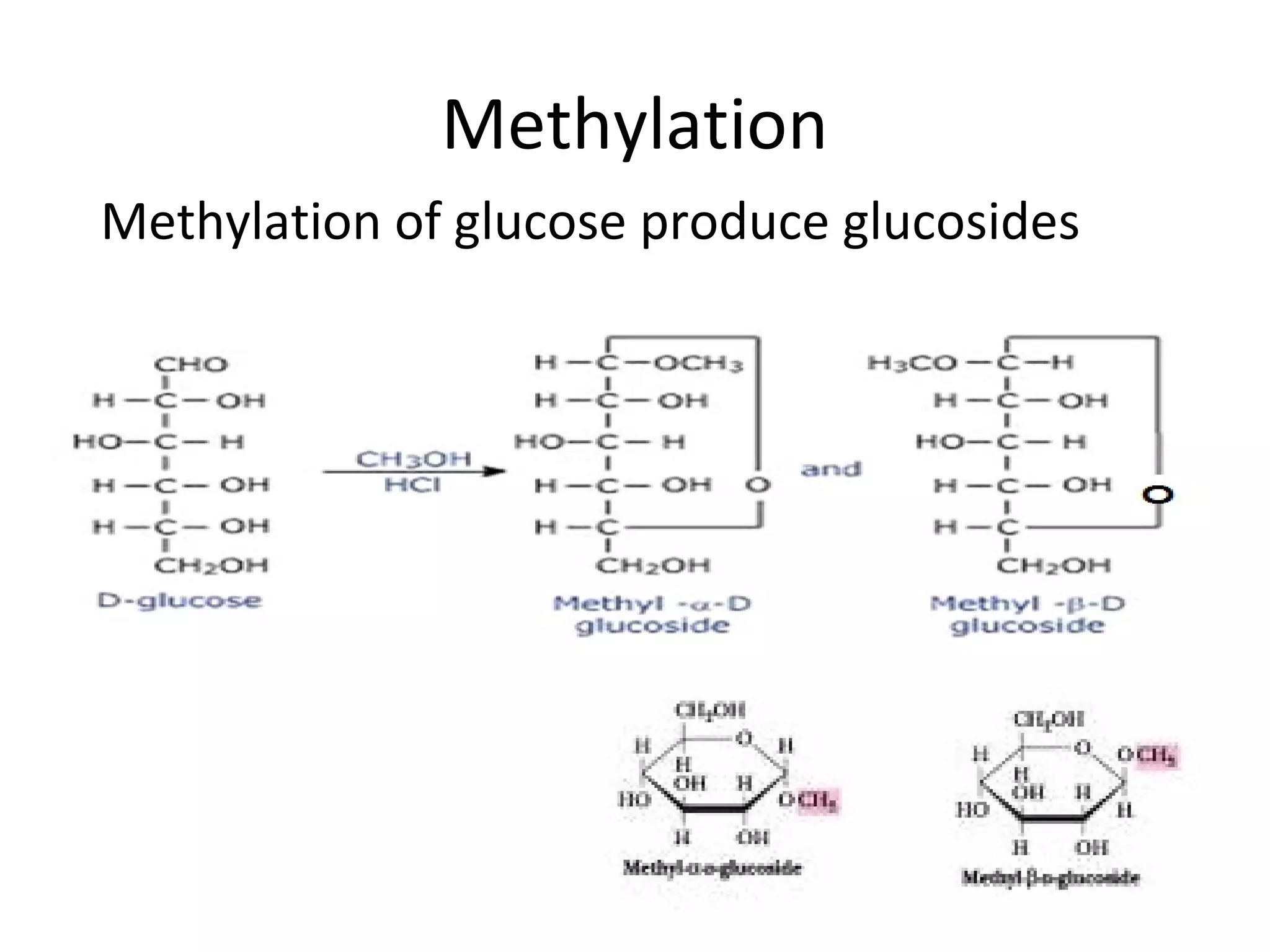
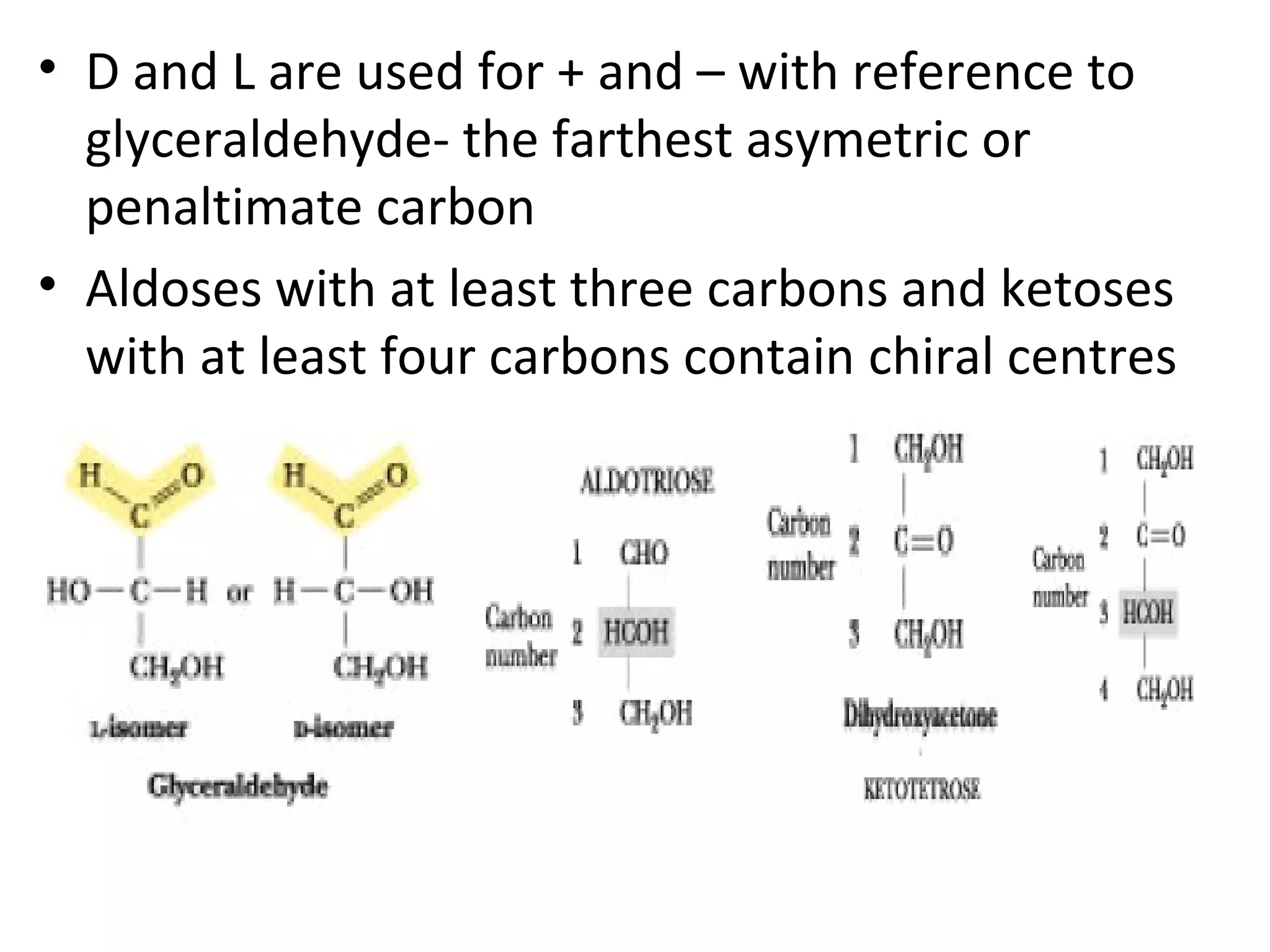
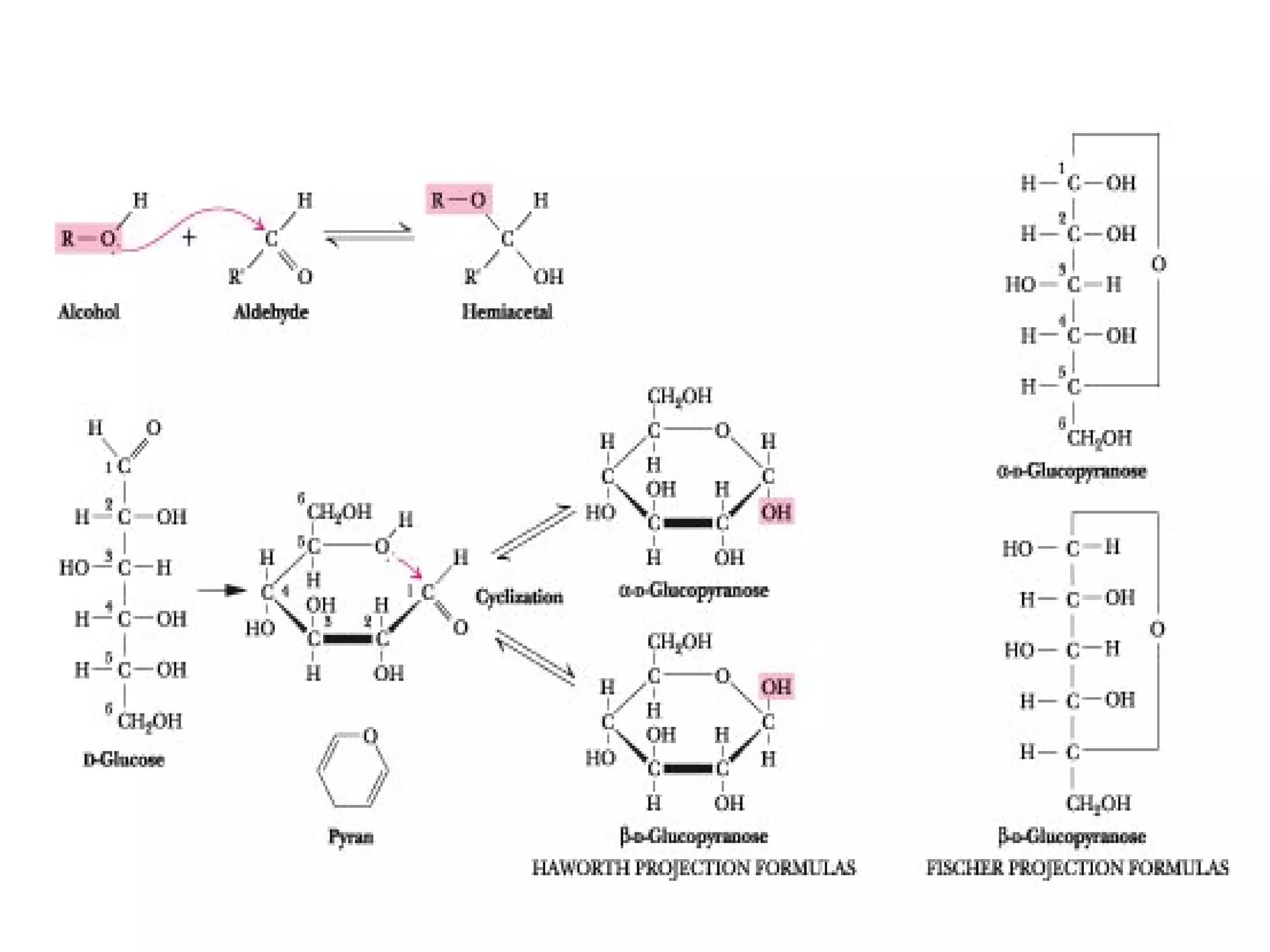
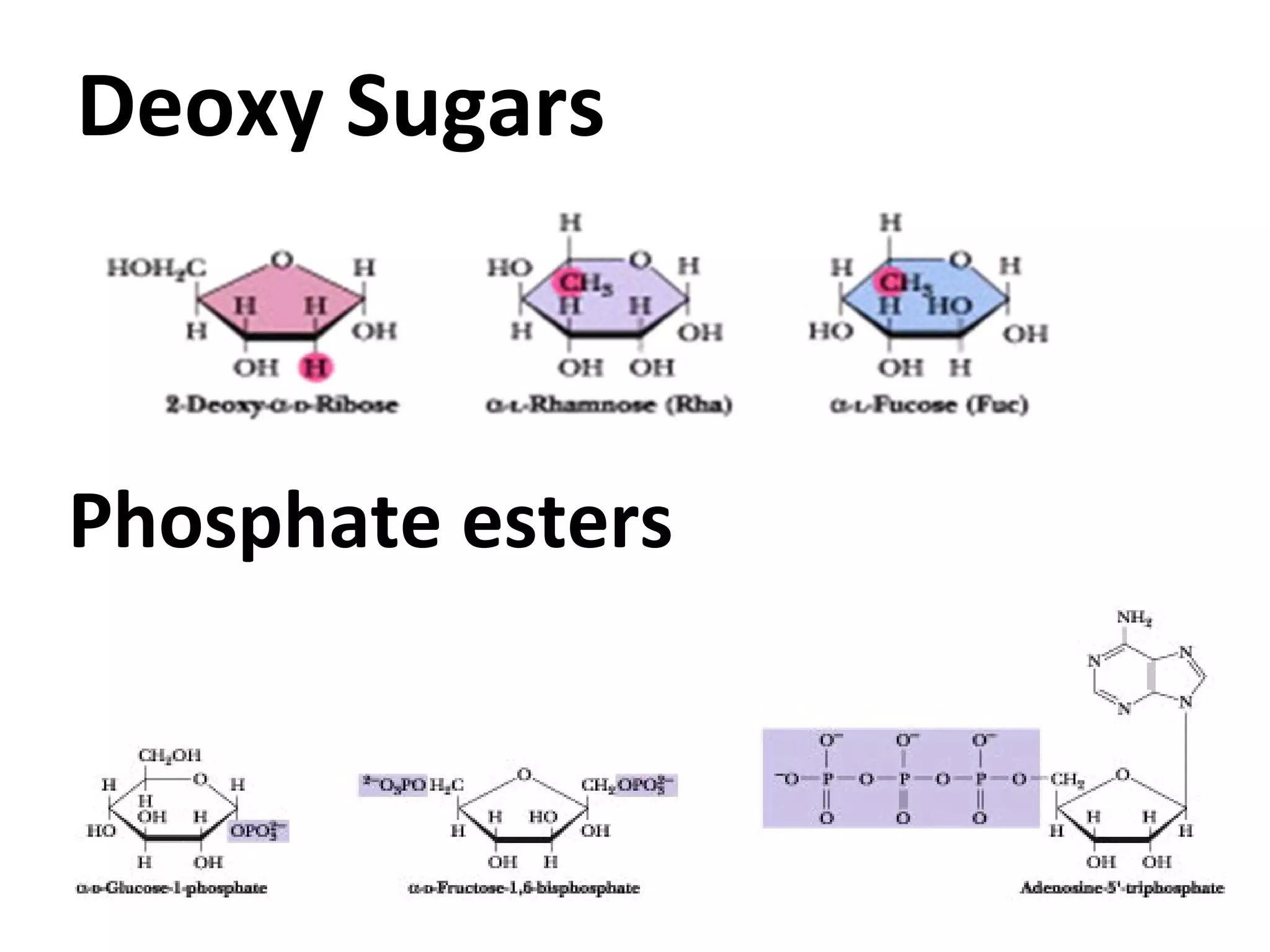
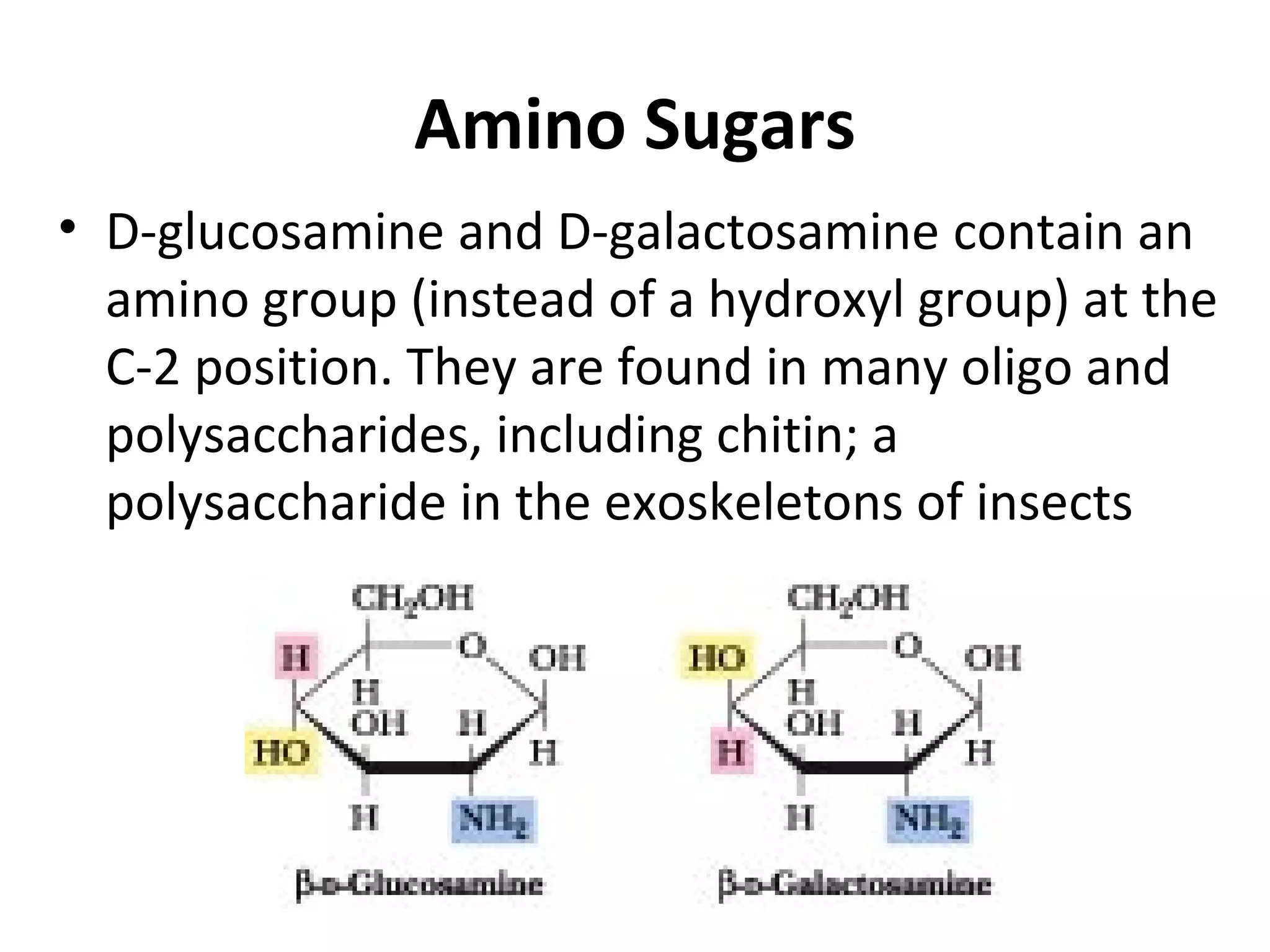
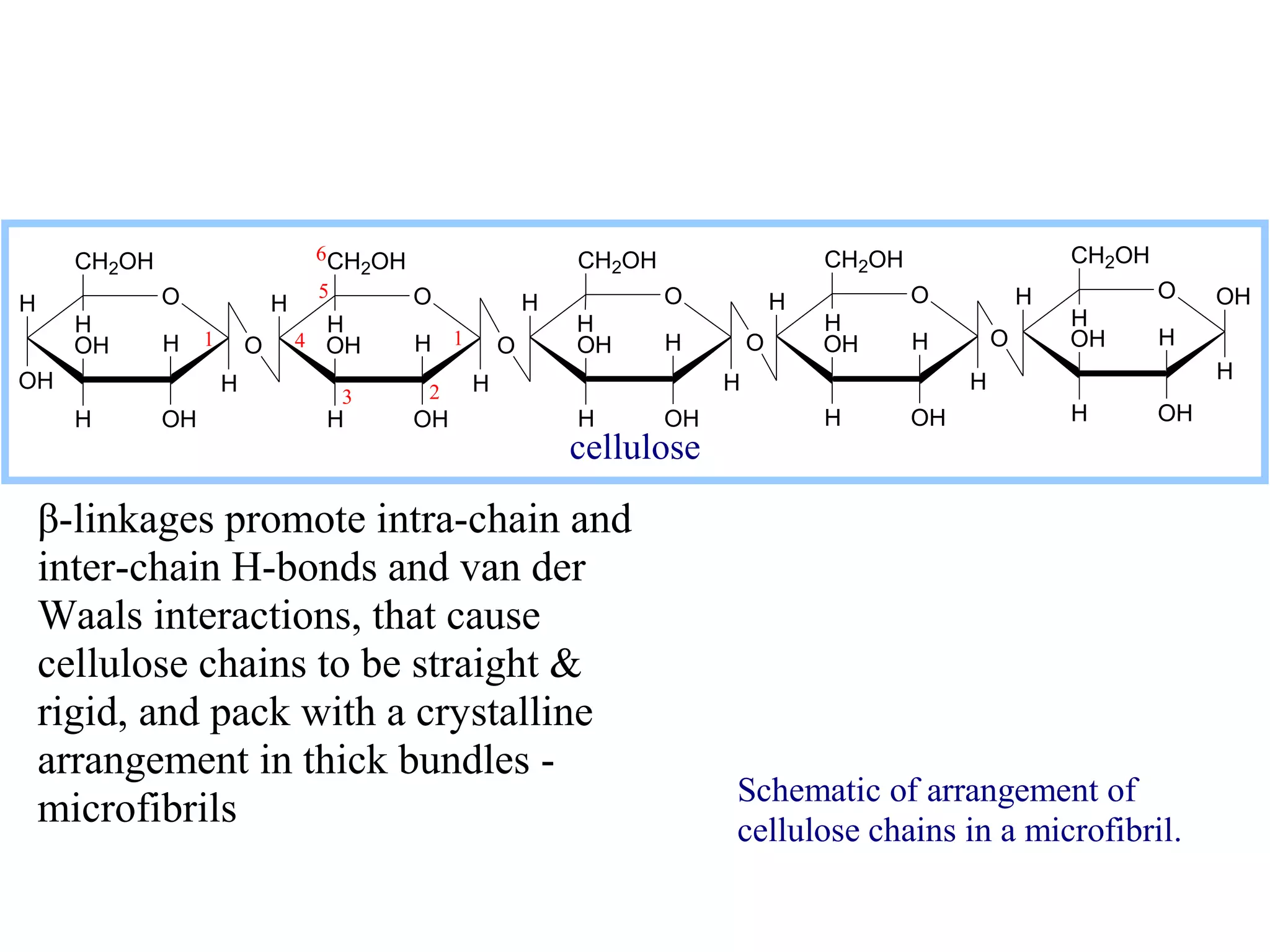
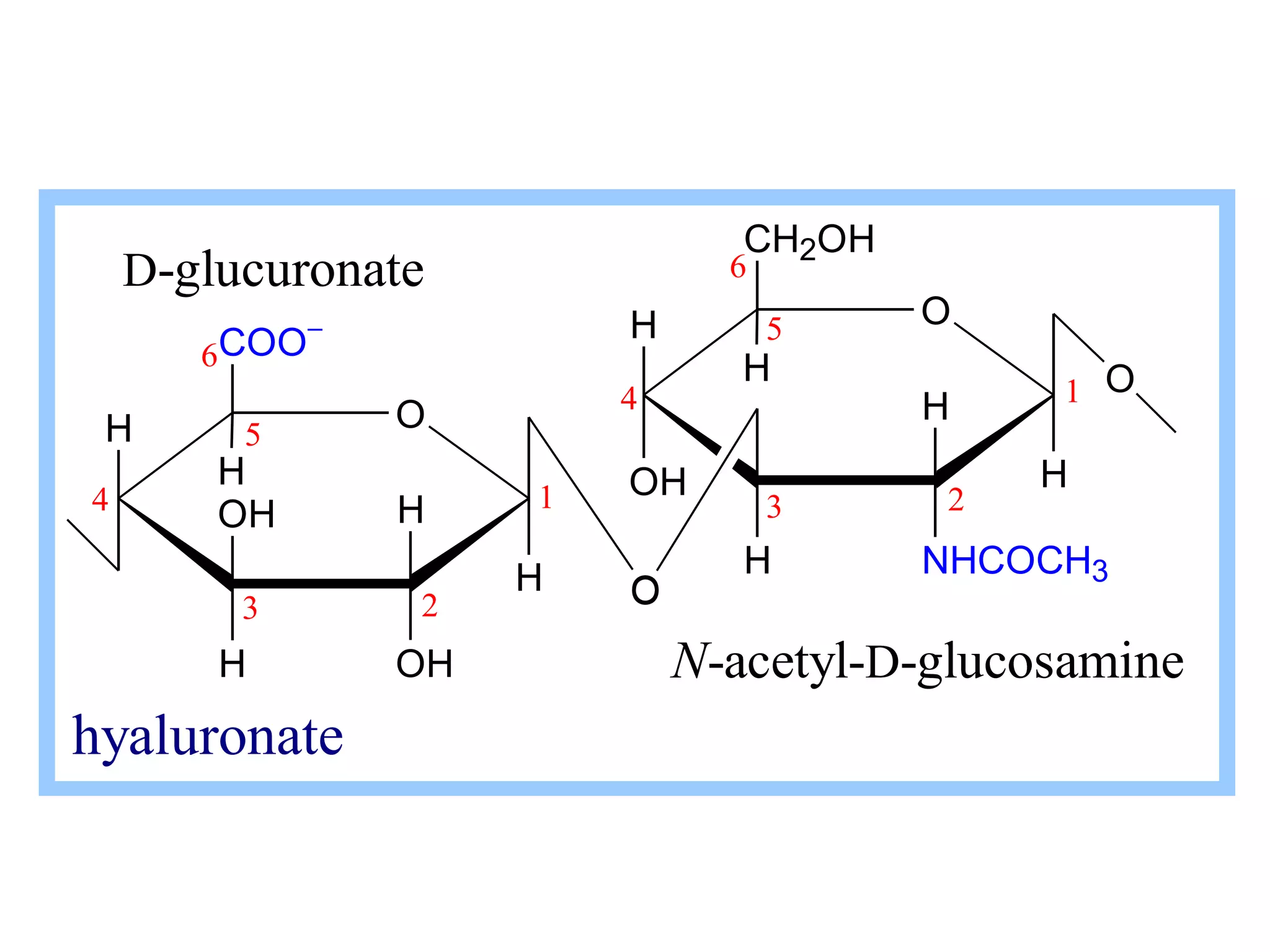
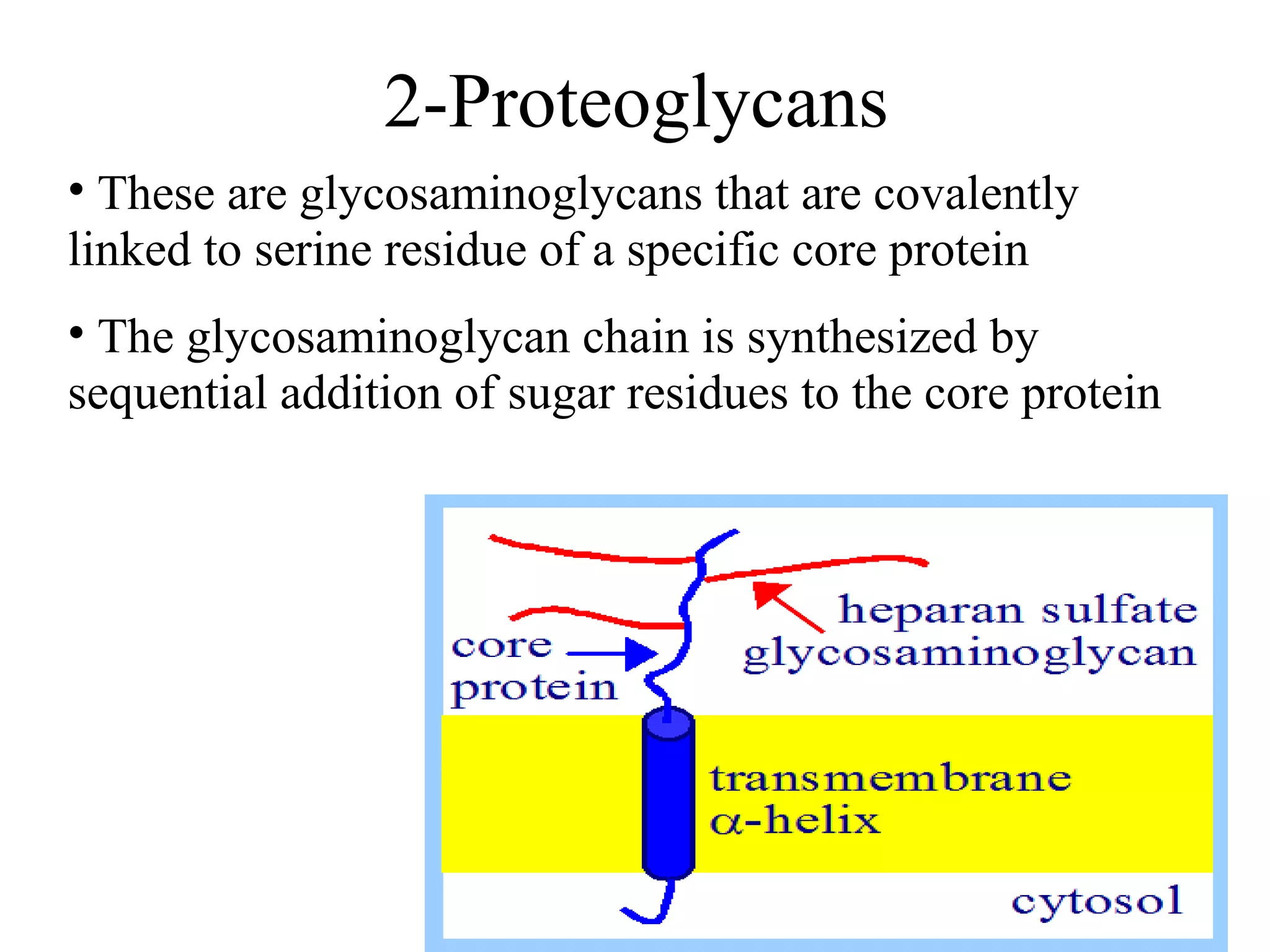
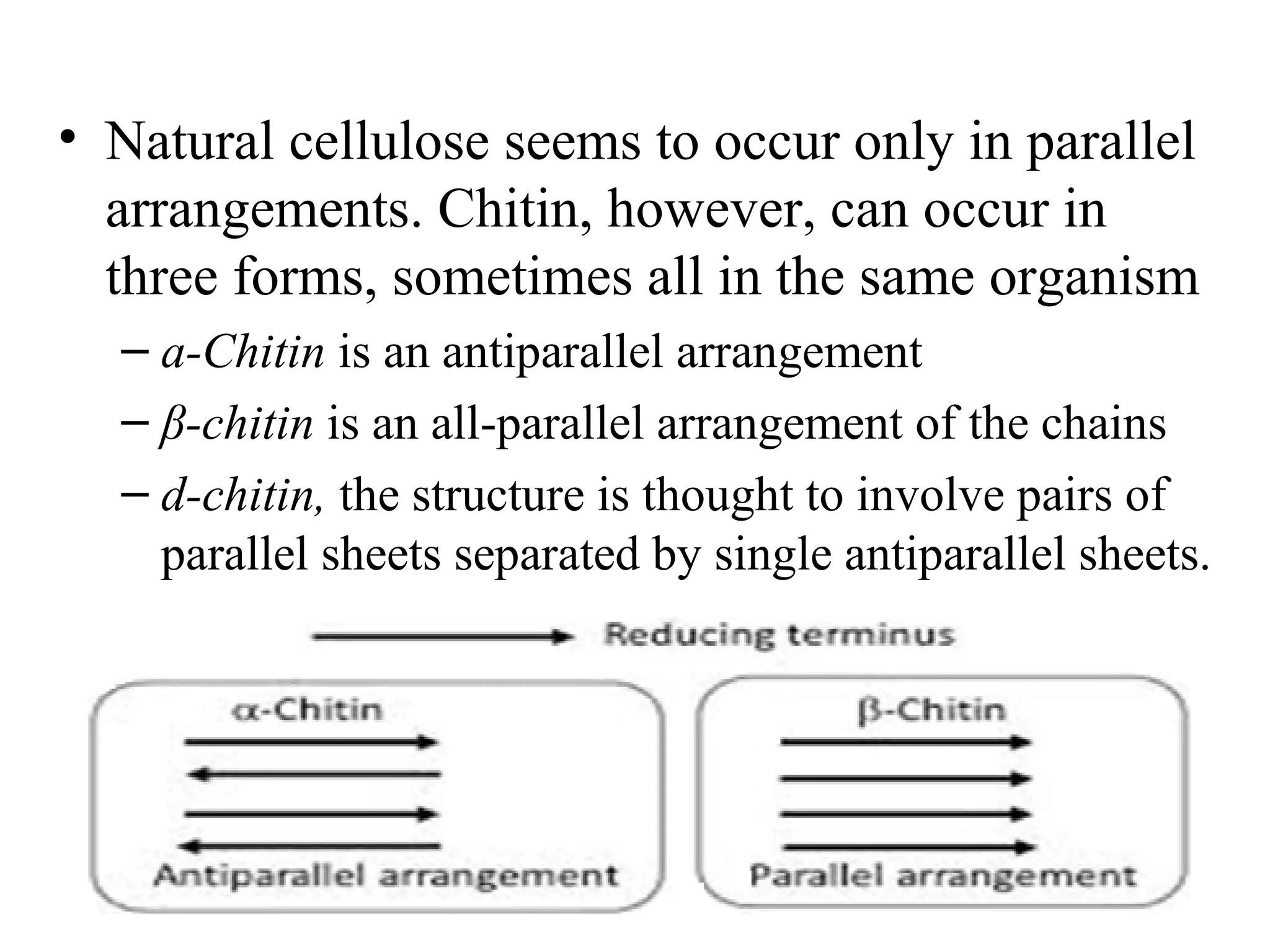
The document discusses various reactions and properties of monosaccharides such as glucose and fructose. It explains that glucose can be converted to sorbitol or mannitol through reduction, and that glucose and fructose can isomerize to each other in weak alkali. It also describes how monosaccharides can cyclize through hemiacetal and hemiketal formation to form pyranoses and furanoses with alpha and beta anomers. Storage polysaccharides like starch, glycogen, and structural polysaccharides such as cellulose, chitin, and glycosaminoglycans are formed from linked monosaccharide units.