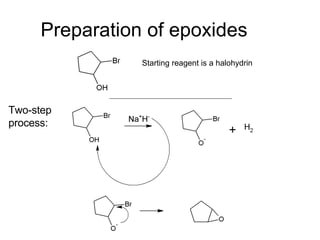
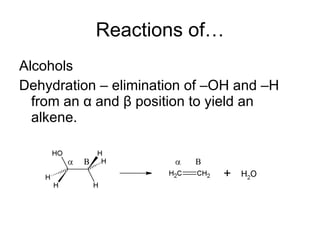
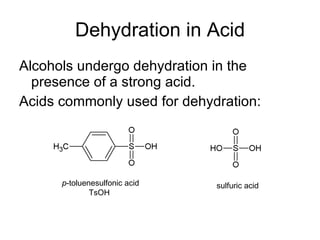
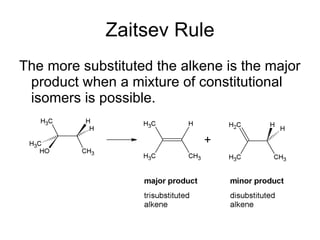
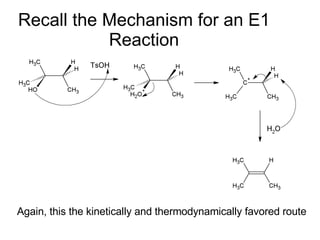
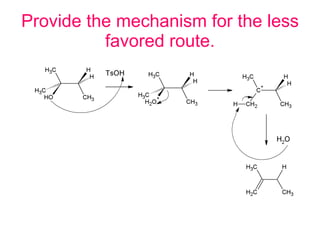
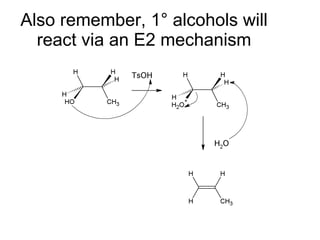
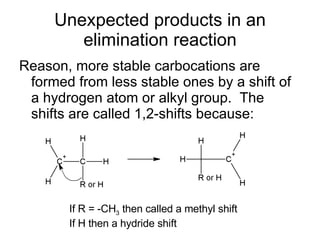
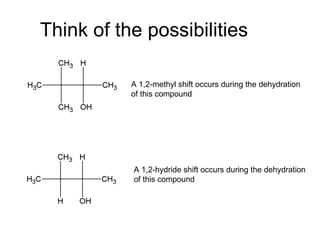
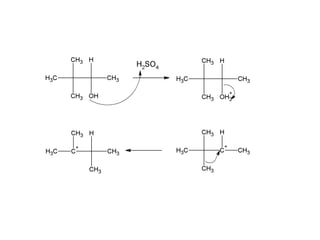
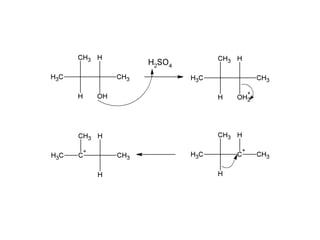
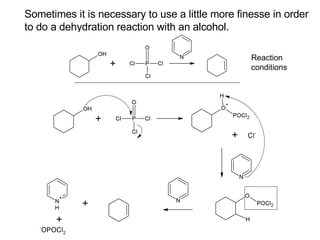
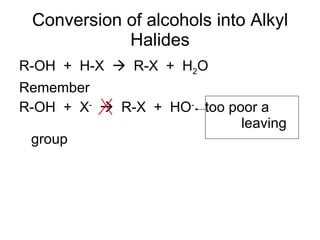
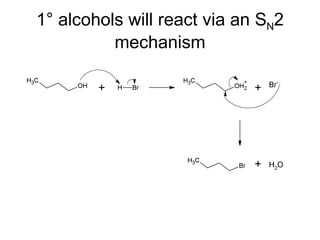
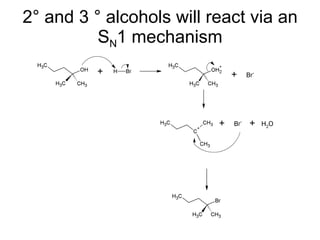
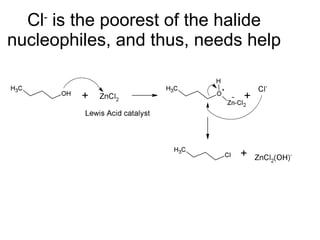
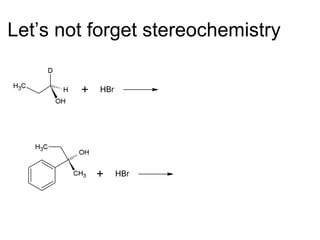
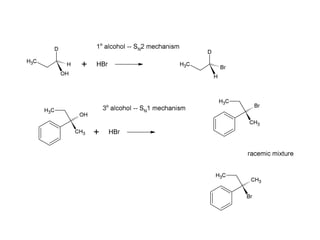
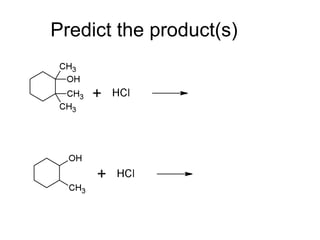
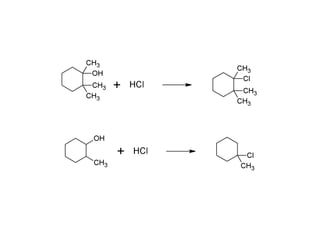
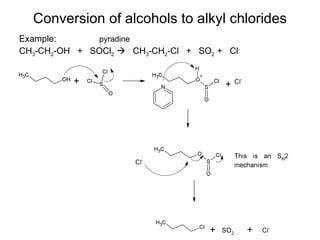
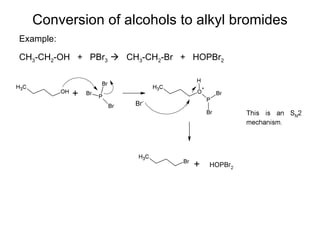
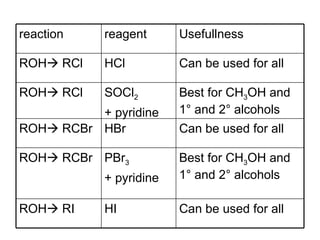
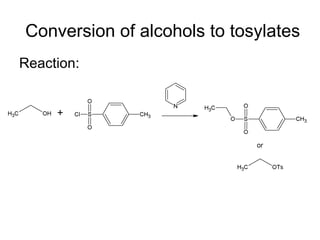
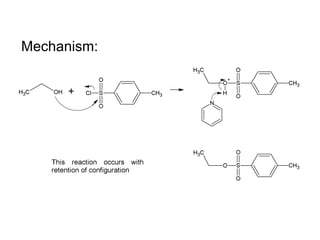
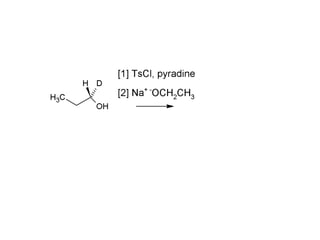
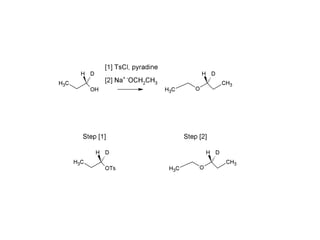
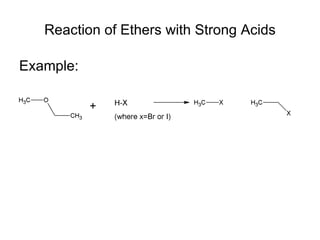
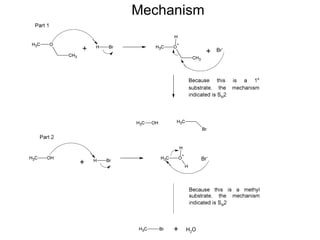
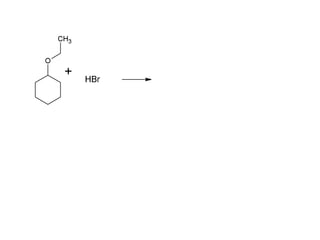
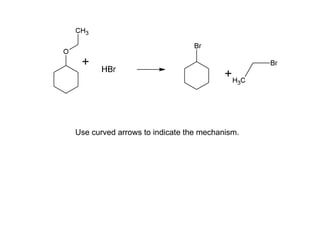
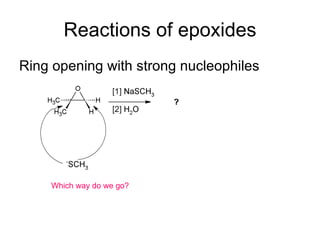
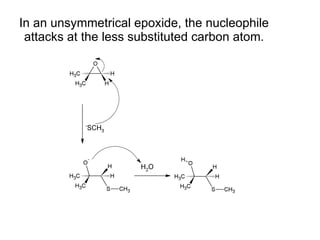
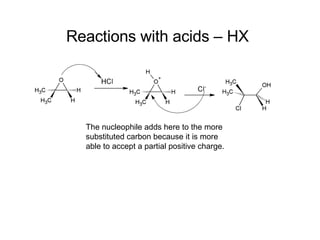
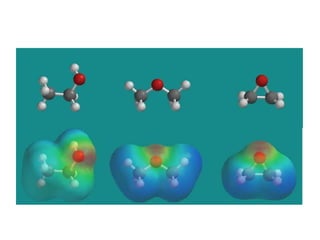
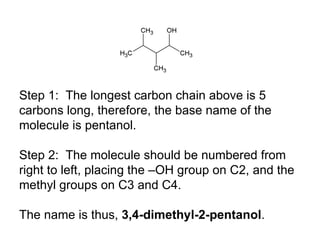
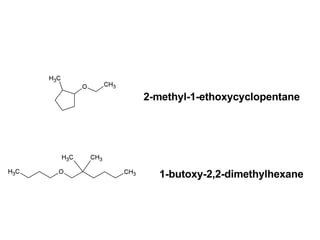
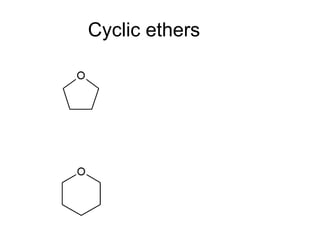
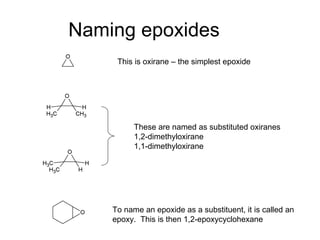
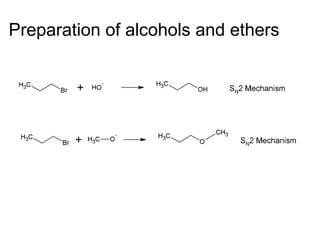
This document discusses IUPAC nomenclature rules for alcohols, ethers, and epoxides. It also summarizes common reactions of alcohols including dehydration, conversion to alkyl halides, and conversion to tosylates. Reactions of ethers with strong acids and epoxide ring opening reactions are also covered.


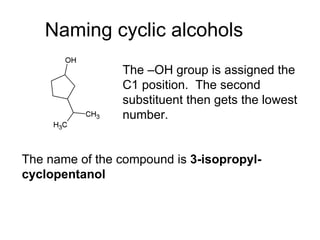
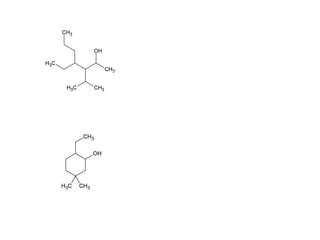
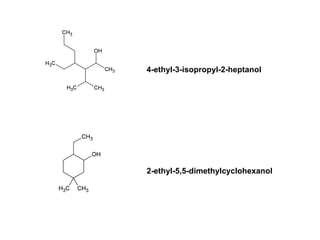
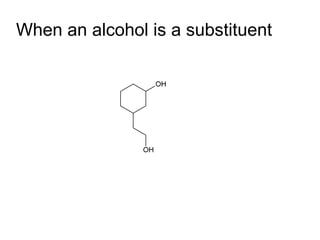
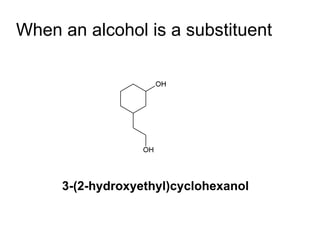

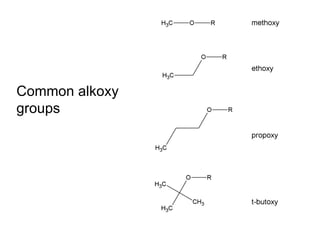
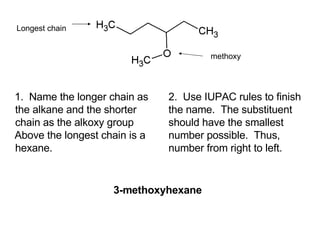
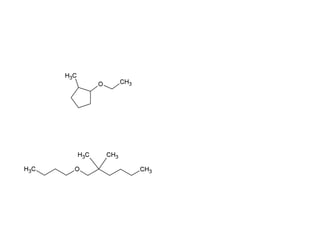
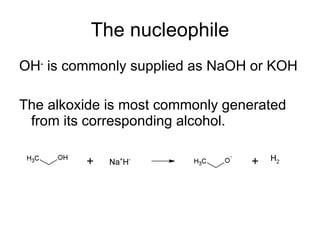
![Draw the product of the following two-step sequence [1] In the first step the base NaH removes the proton from the alcohol [2] In the second step of the process the alkoxide acts as a nucleophile displacing the leaving group in an S N 2 reaction](https://image.slidesharecdn.com/chapter9-090515122343-phpapp02/85/Chapter9-19-320.jpg)