1. The human body is composed of biomolecules like carbohydrates, proteins, vitamins, and nucleic acids. These biomolecules interact and allow life processes in cells, tissues, organs and the whole living organism.
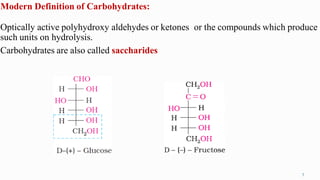
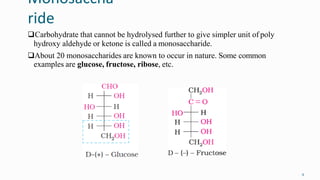
2. Carbohydrates play important roles in the human body, providing energy and performing structural functions. Genetic information is transferred from parents to offspring through DNA and RNA.
3. A balanced diet with proteins, carbohydrates, vitamins and minerals is important for human health and well-being.