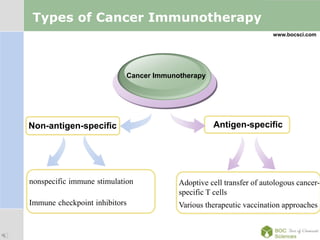
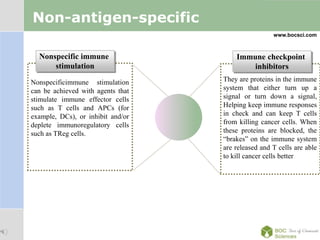
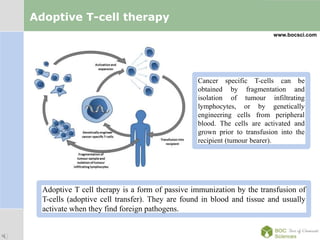
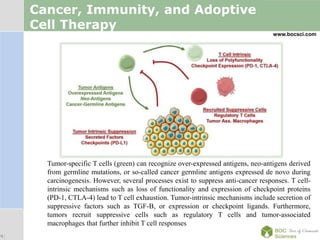
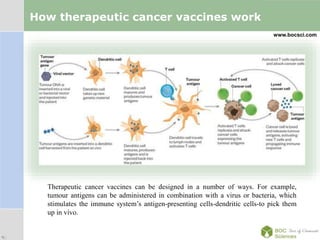
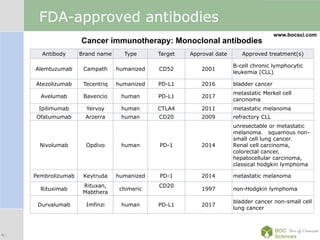
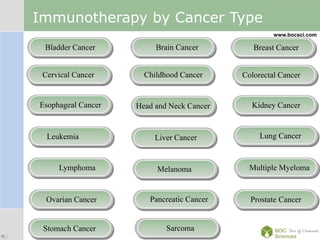
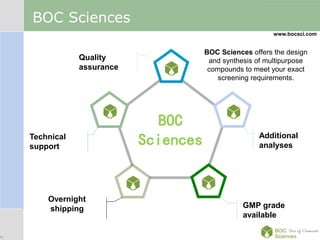
Cancer immunotherapy, also known as immuno-oncology, enhances the immune system's natural ability to combat cancer through various methods, including both nonspecific immune stimulation and antigen-specific approaches. FDA-approved therapies include immune checkpoint inhibitors, adoptive cell transfer, and therapeutic vaccines targeting different cancers. The document details specific antibodies approved for treatment, types of immunotherapies, and the cancer types that can benefit from these treatments.