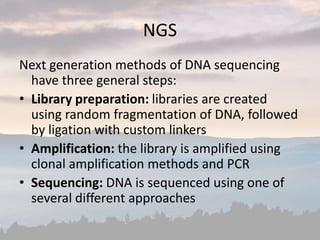
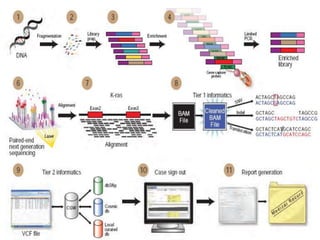
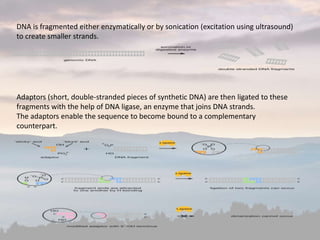
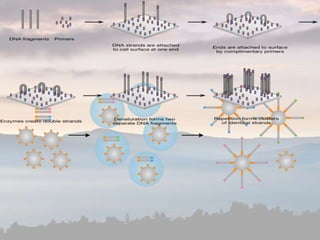
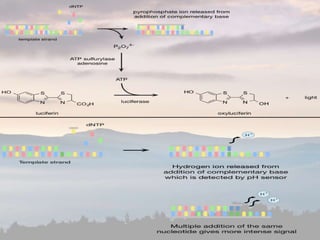
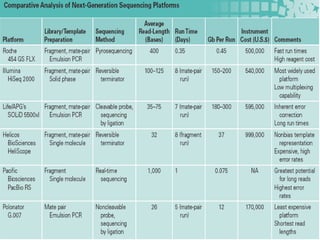
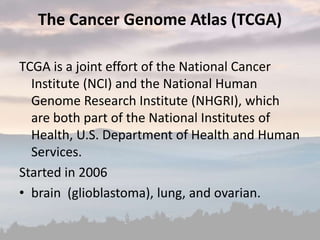
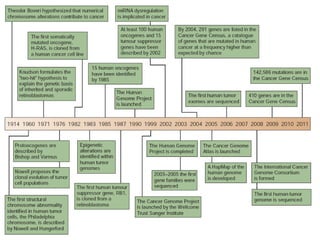
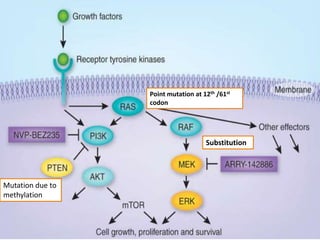
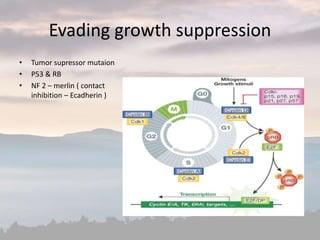
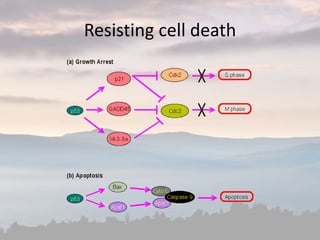
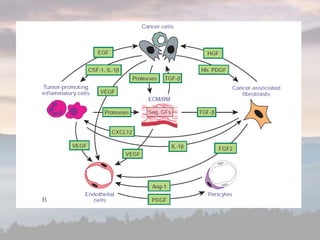
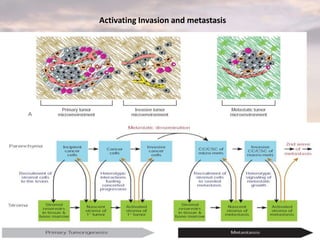
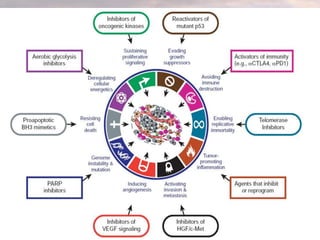
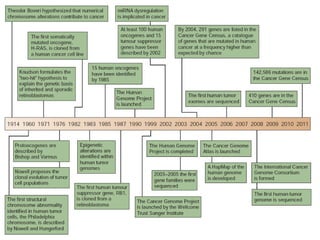
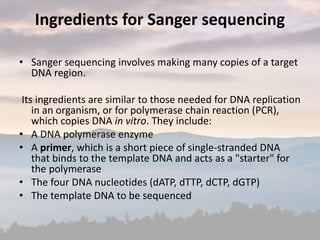
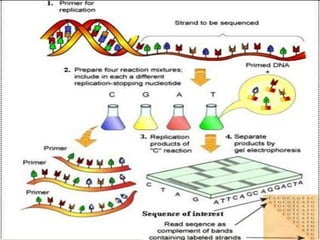
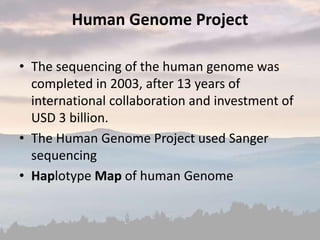
This document discusses cancer genomics and tumor sequencing. It explains that tumor genotyping helps clinicians individualize cancer treatments by matching patients to the best treatment based on their tumor's DNA alterations. Next generation sequencing methods have made it possible to sequence entire cancer genomes and identify additional targets for new cancer therapies. Large-scale projects like The Cancer Genome Atlas and the International Cancer Genome Consortium are analyzing hundreds of cancer genomes to better understand the molecular changes driving different cancer types.




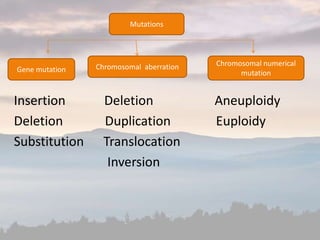











![• A single-nucleotide polymorphism (SNP,
pronounced snip) is a DNA sequence variation
occurring when a single nucleotide adenine
(A), thymine (T), cytosine (C), or guanine (G])
in the genome (or other shared sequence)
differs between members of a species or
paired chromosomes in an individual.](https://image.slidesharecdn.com/cancergenome-180624161119/85/Cancer-genome-38-320.jpg)