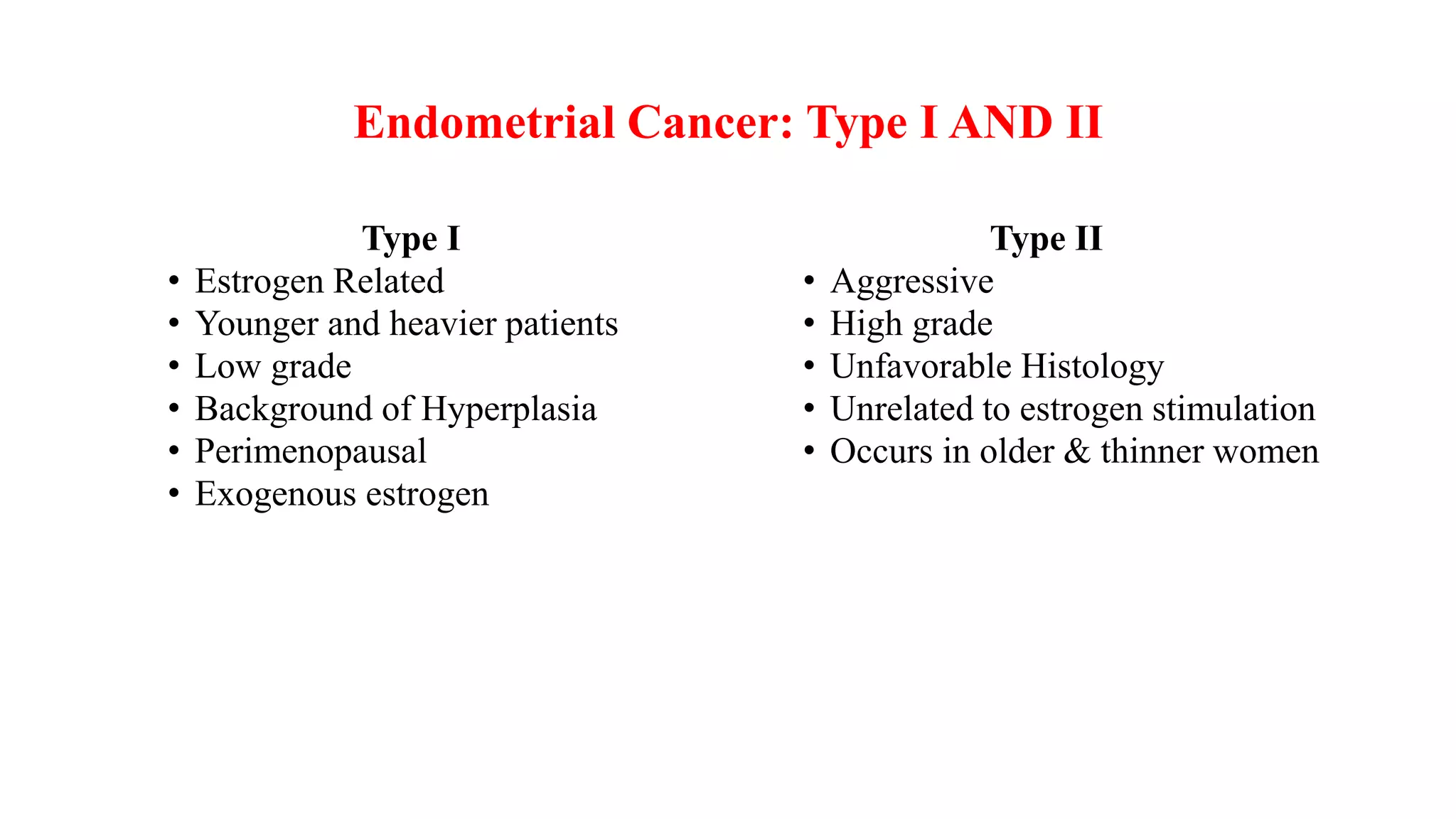
1) Endometrial cancer is the most common gynecologic cancer and risk increases with factors like postmenopausal bleeding, obesity, diabetes, and unopposed estrogen use.
2) Diagnostic workup includes endometrial biopsy or D&C followed by surgical staging including TAH/BSO and lymph node assessment.
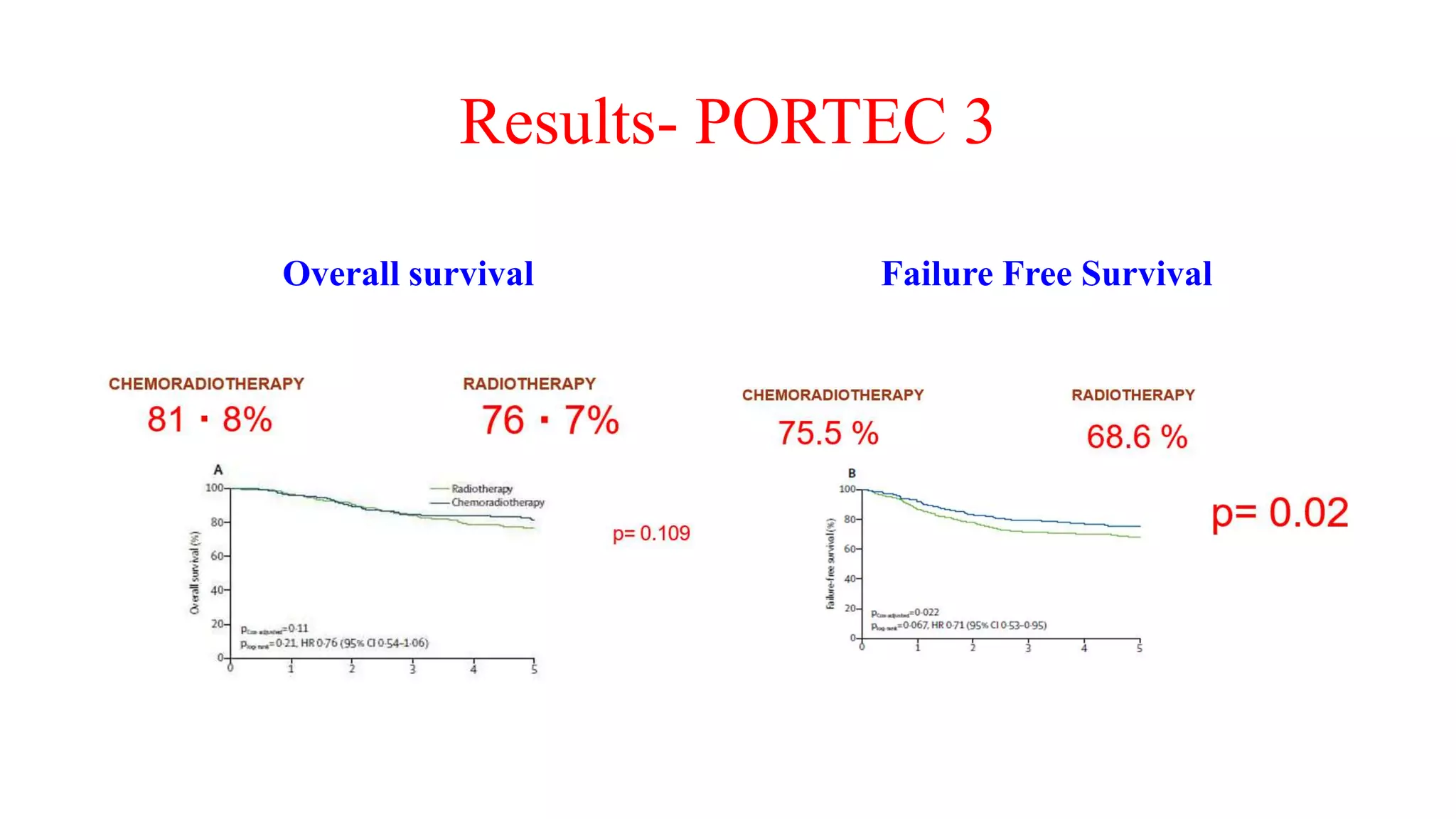
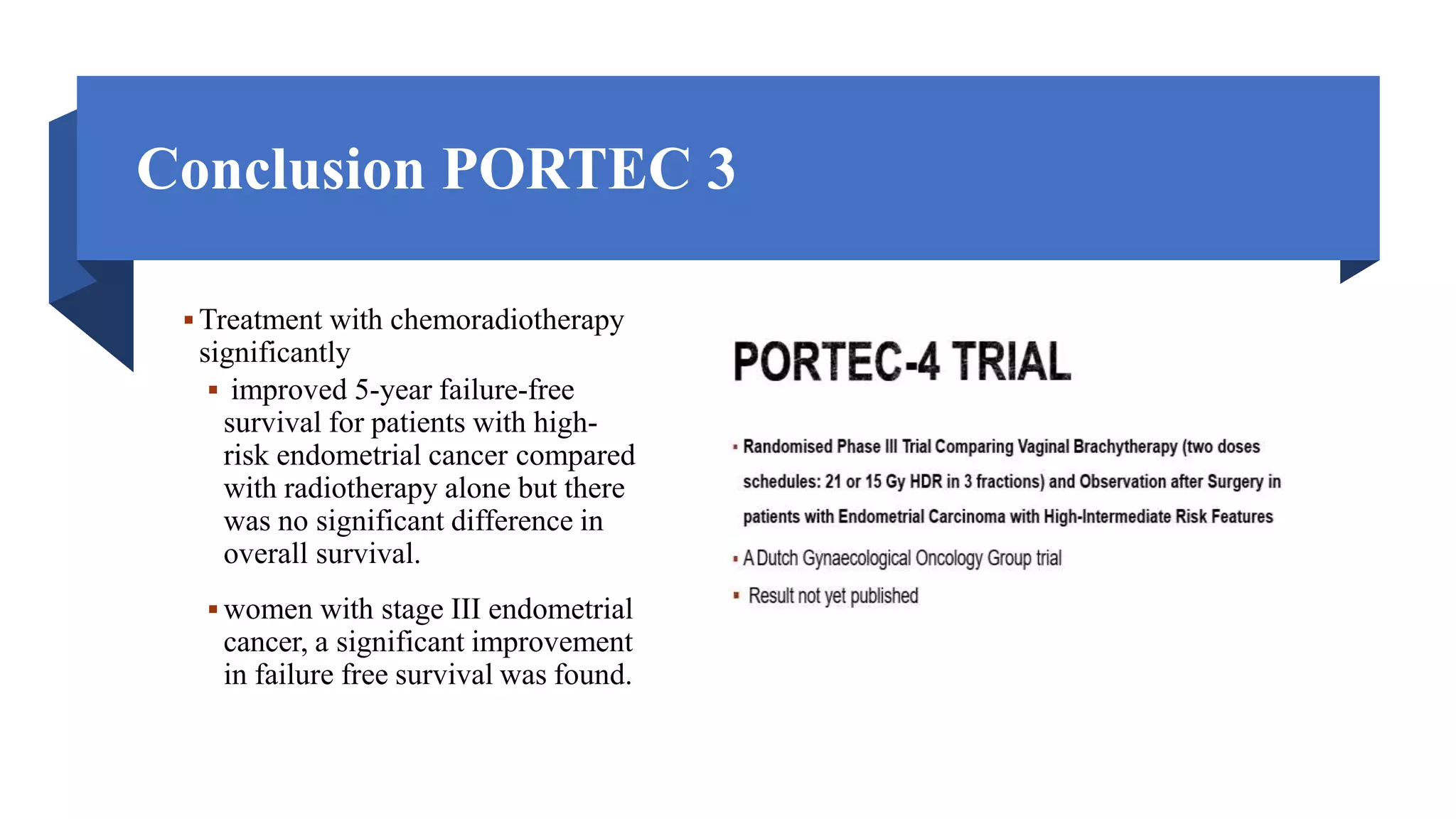
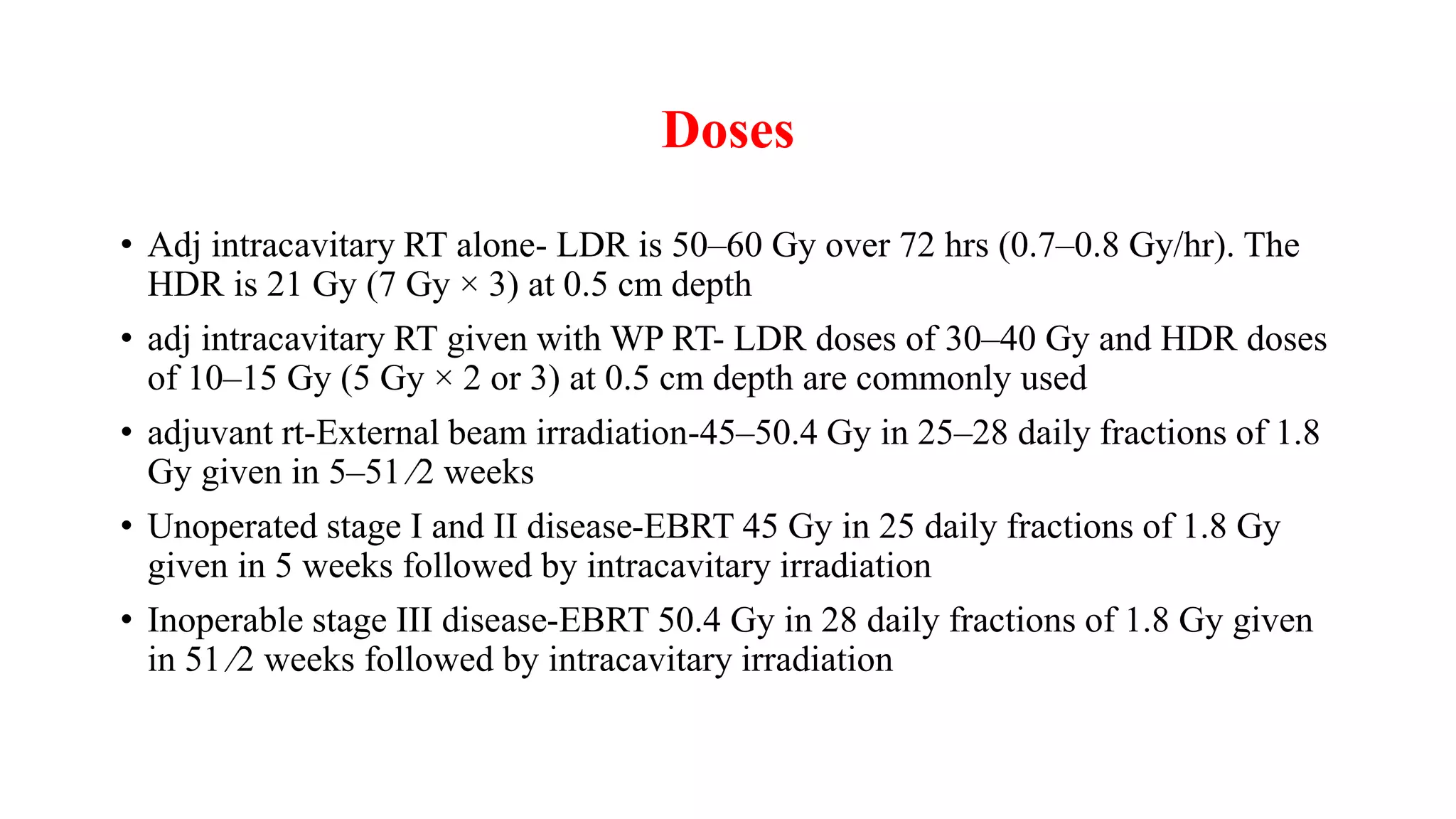
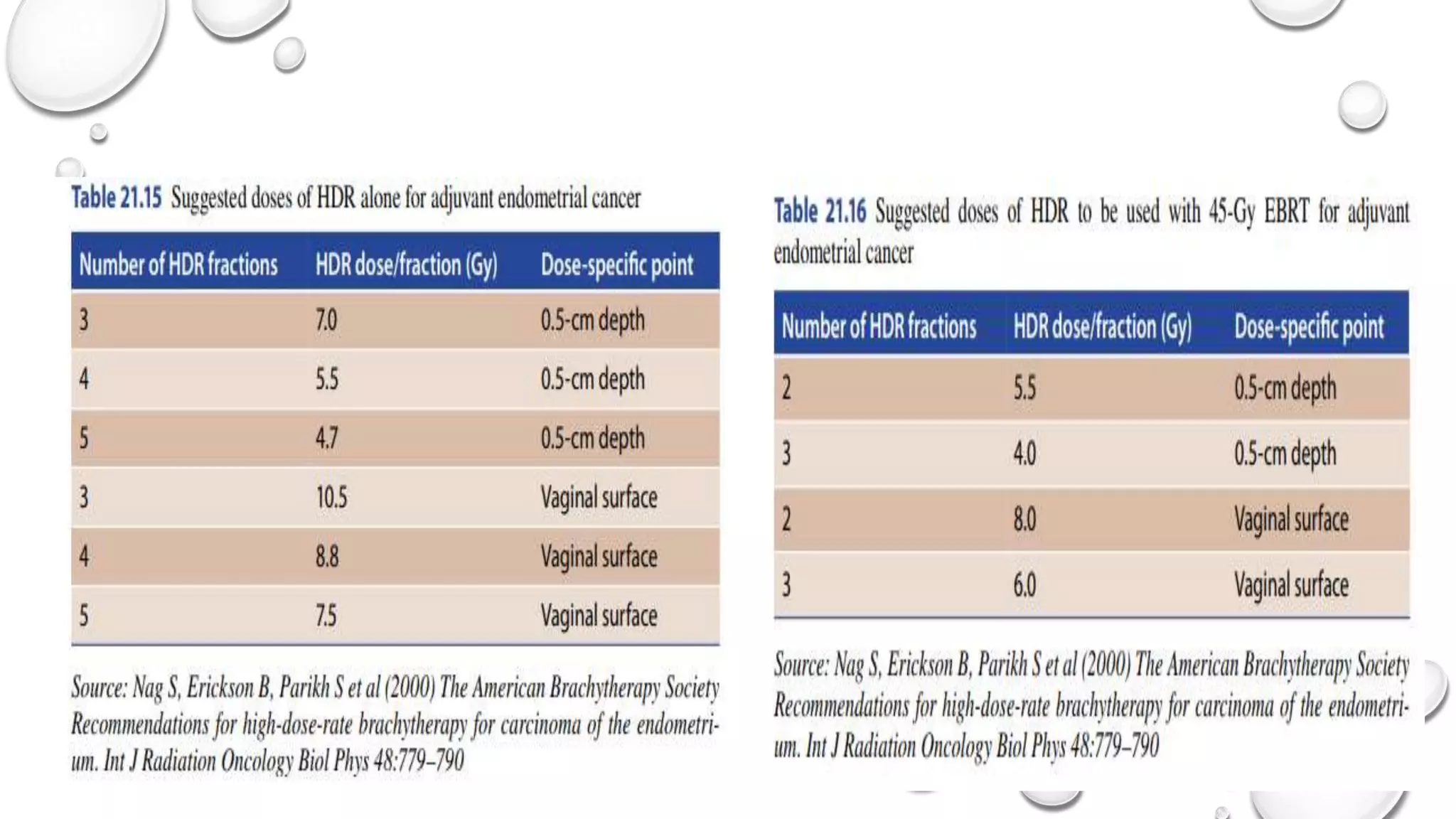
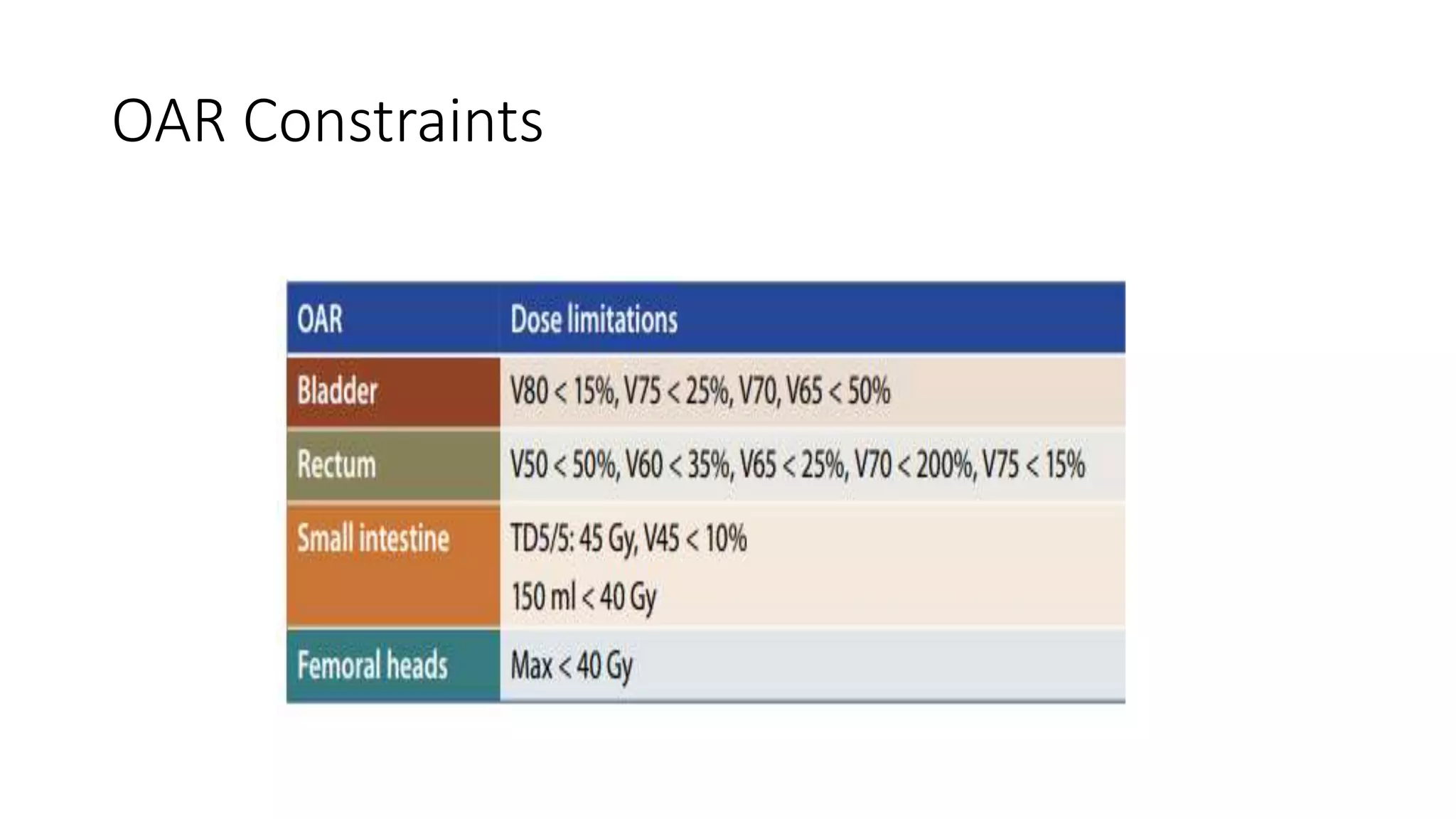
3) Treatment depends on surgical staging - low risk receives no additional treatment; intermediate receives vaginal brachytherapy; high risk receives pelvic radiation with concurrent chemotherapy based on GOG-249 trial results showing improved outcomes.










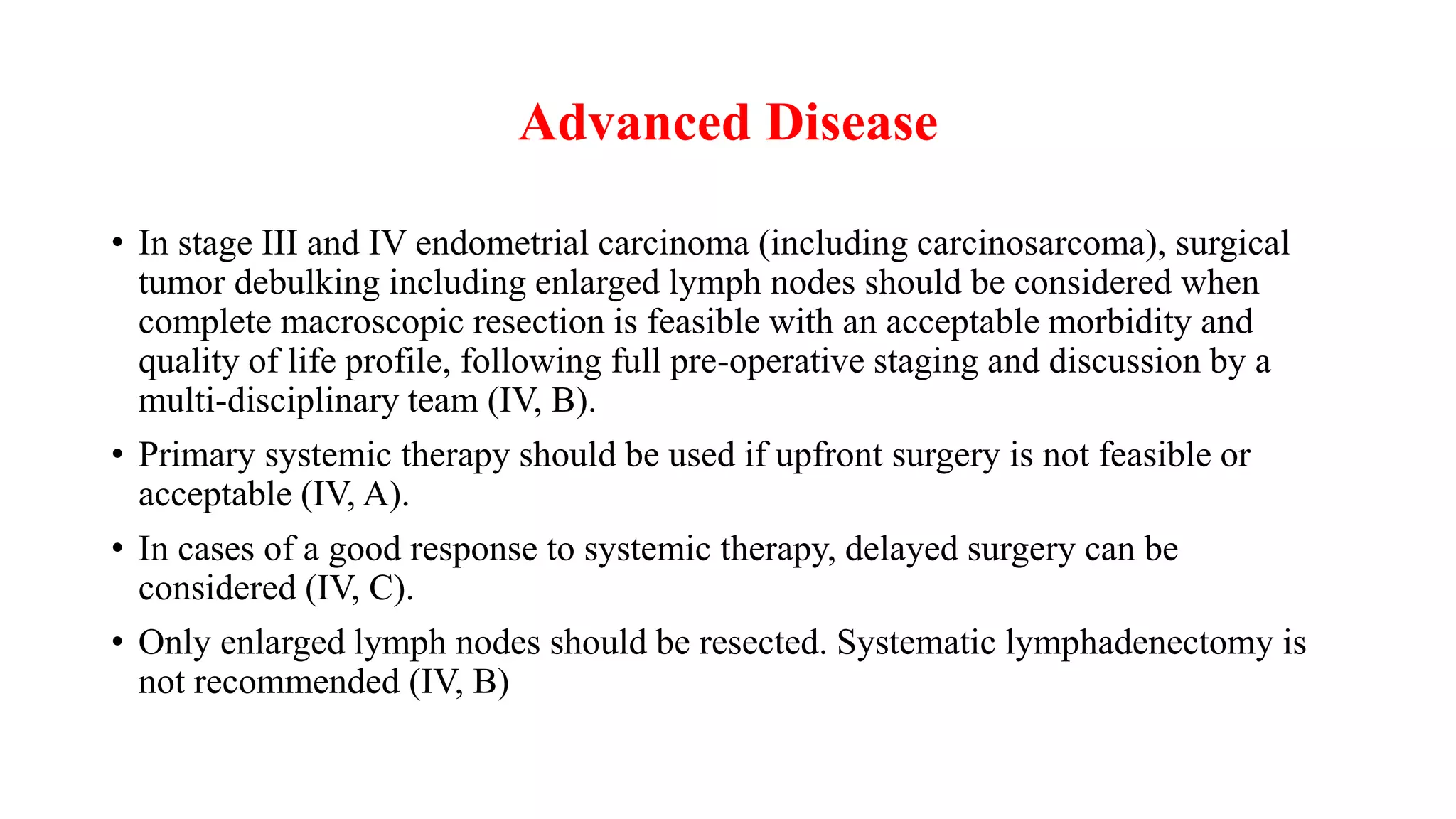
![Surgical Assessment of Lymph Nodes
• Lymphadenectomy-limit nodal assessment to
inspection and removal of any
enlarged/suspicious pelvic or paraaortic nodes.
• Lack of documented survival advantages to
lymphadenectomy.
• full-pelvic and para-aortic lymph node
sampling-surgical staging is the most accurate
method to assess the extent of disease.
• Sentinel lymph node biopsy
Medical Research Council [MRC]/A Study in the Treatment of Endometrial Cancer [ASTEC]](https://image.slidesharecdn.com/caendometrium-230805172835-ce65b4f8/75/CA-ENDOMETRIUM-pptx-12-2048.jpg)











![ Given in both treatment group
Total dose 48.6 Gy 1.8 Gy fractions , 5 days a week
In case of cervical involvement (glandular, stromal, or both), a brachytherapy boost was given to
the vaginal vault.
Brachytherapy dose was equivalent to 14 Gy in 2 Gy fractions (with recommended scheme of 10
Gy high-dose rate [HDR] in fractions of 5 Gy), specified at 5 mm from the vaginal vault surface.
• Two cycles of intravenous cisplatin 50 mg/m2 in the first and fourth week of external beam pelvic
radiotherapy, followed by four cycles of intravenous carboplatin AUC5 and paclitaxel 175 mg/m2
at 21-day intervals
TREATMENT](https://image.slidesharecdn.com/caendometrium-230805172835-ce65b4f8/75/CA-ENDOMETRIUM-pptx-26-2048.jpg)