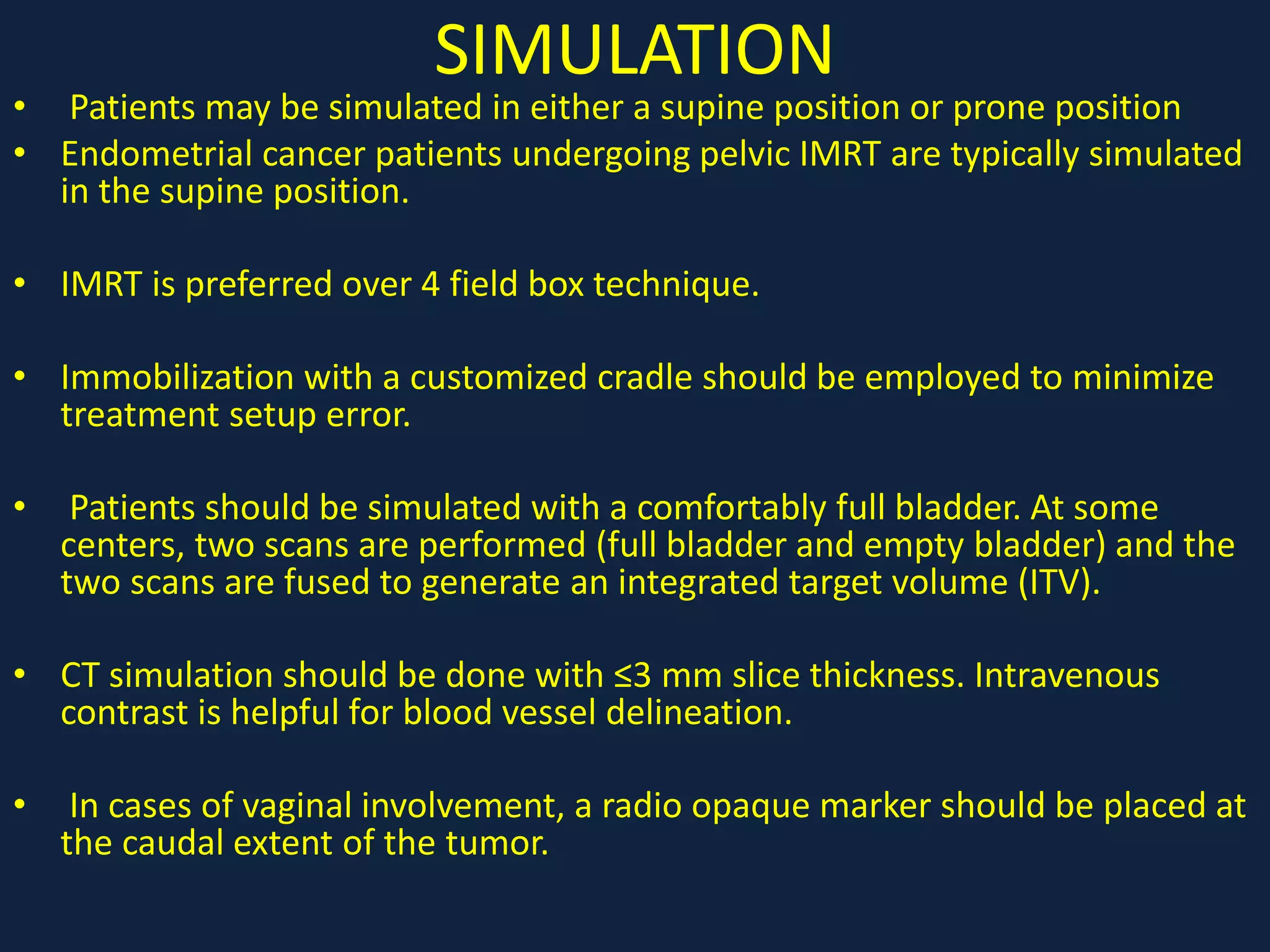
1) Carcinoma endometrium is the most common gynecologic malignancy in Western countries. Risk factors include obesity, early menarche, late menopause, nulliparity, and use of tamoxifen or combined oral contraceptives.
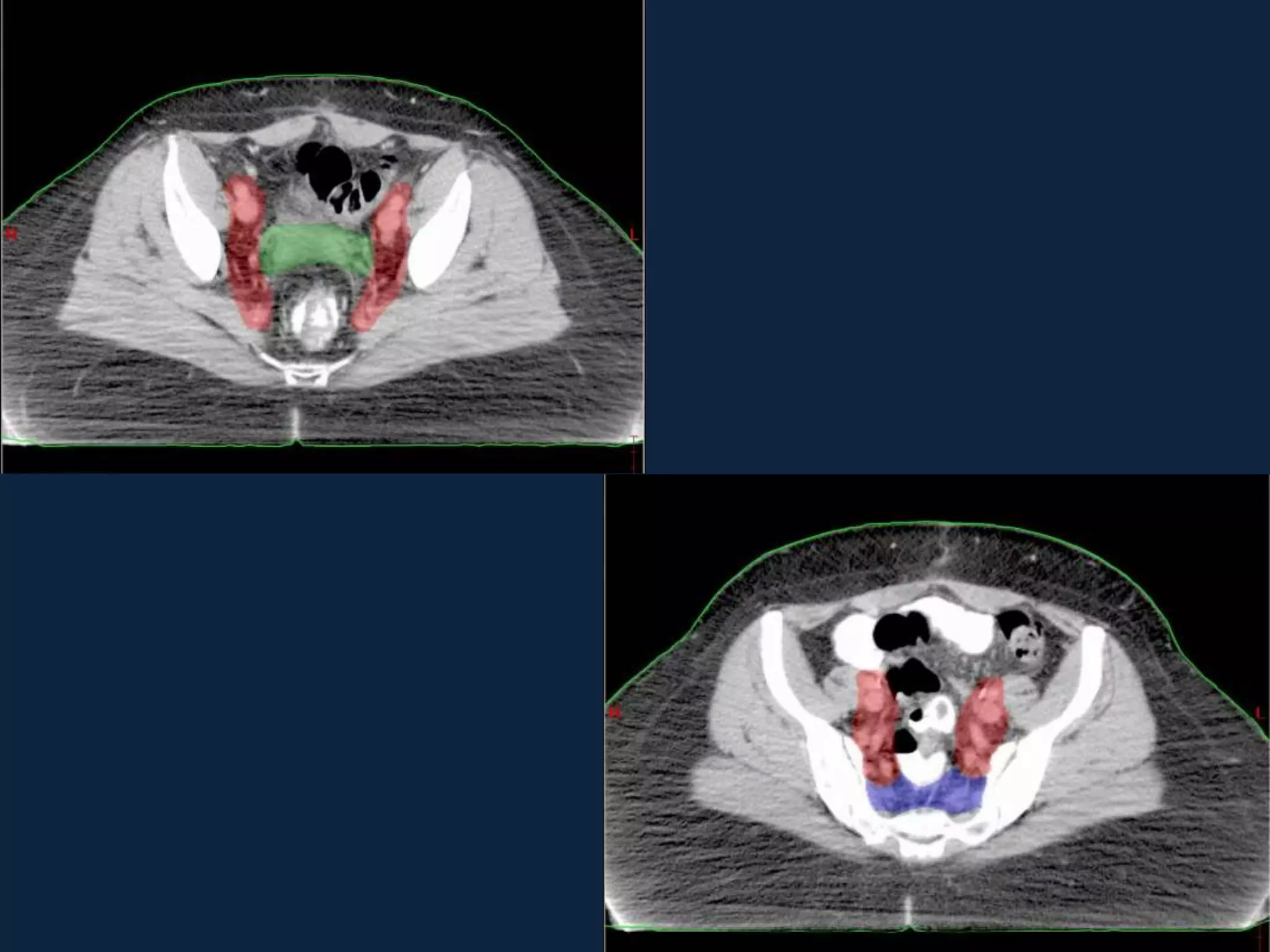
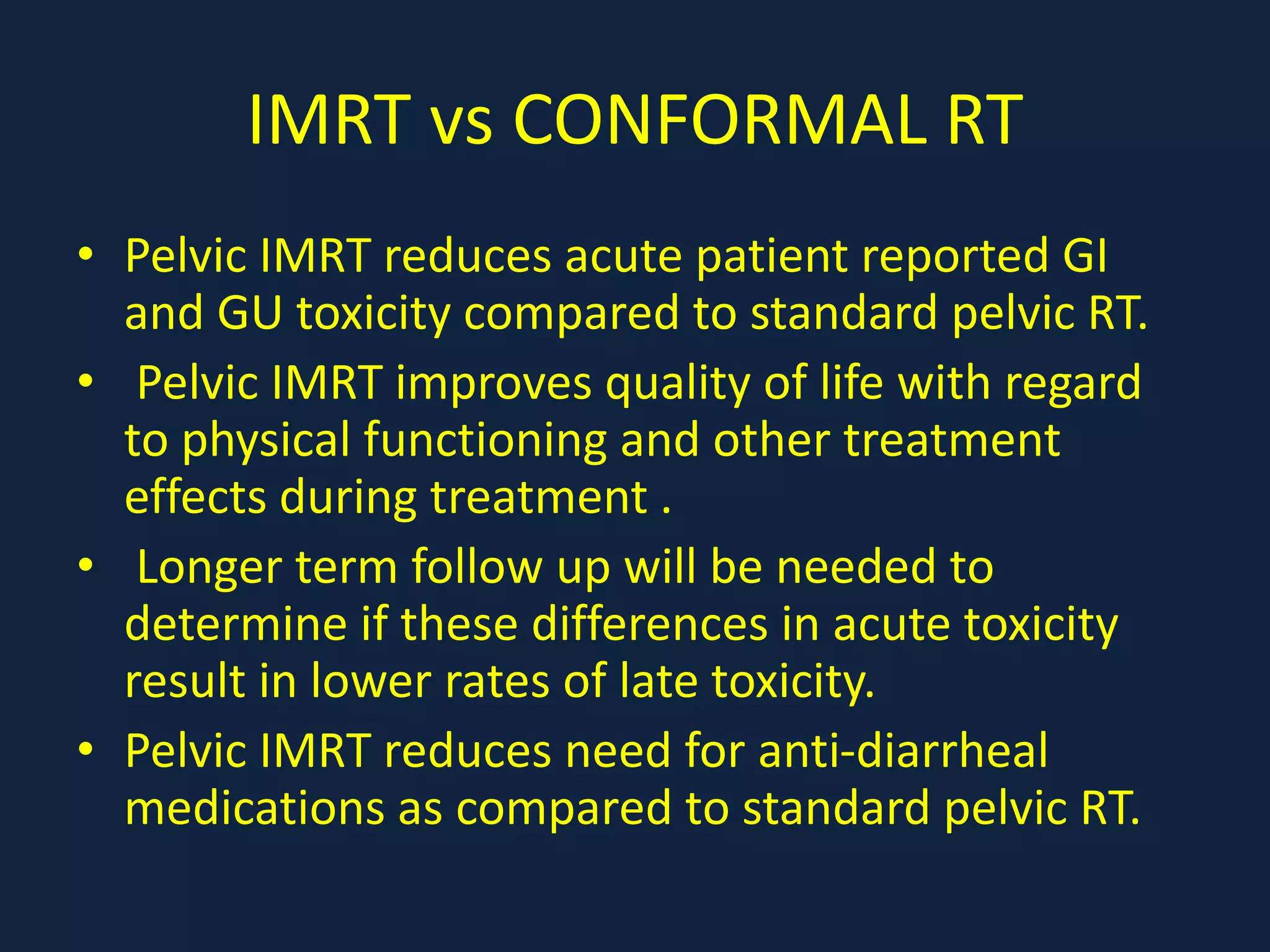
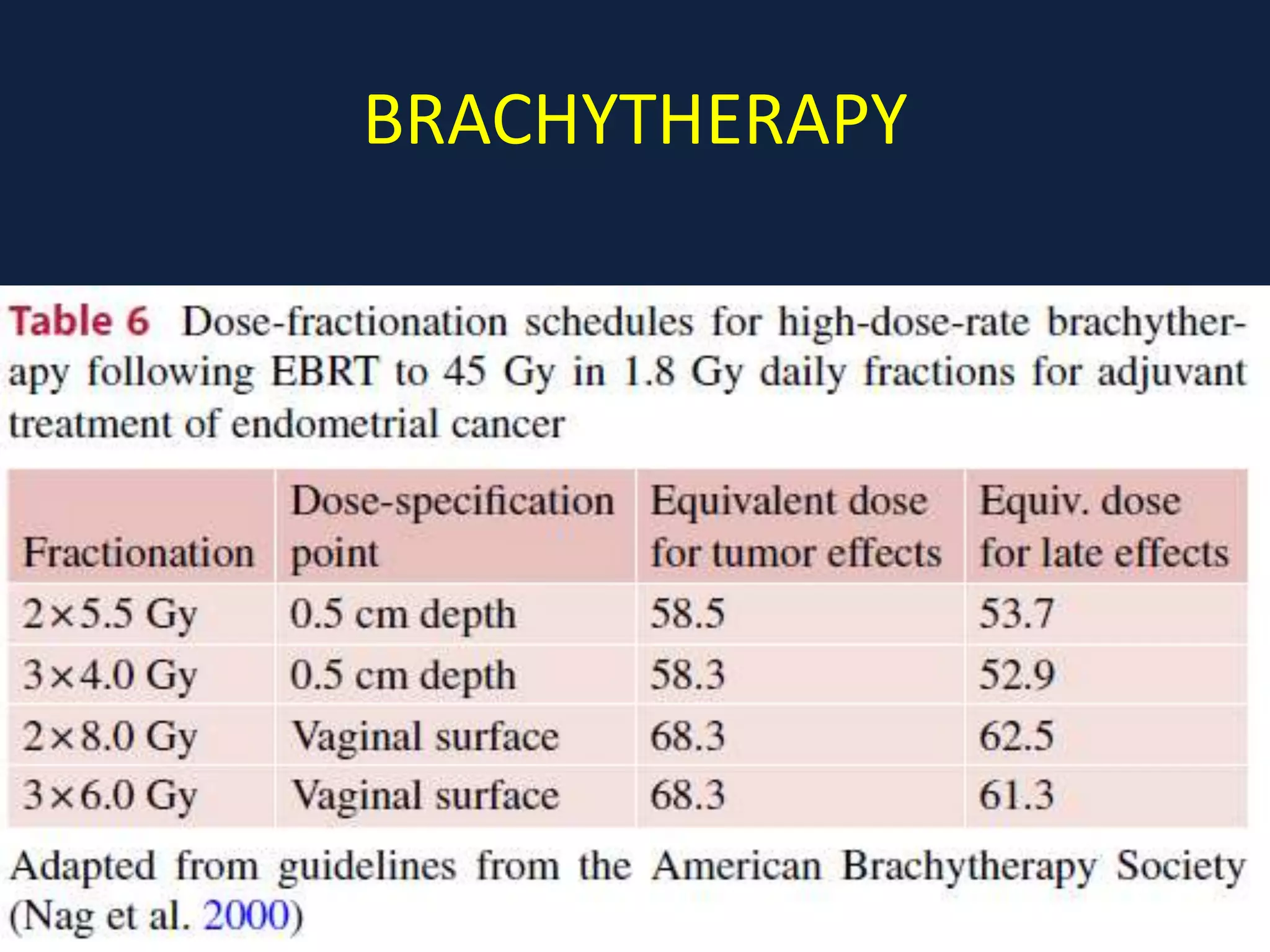
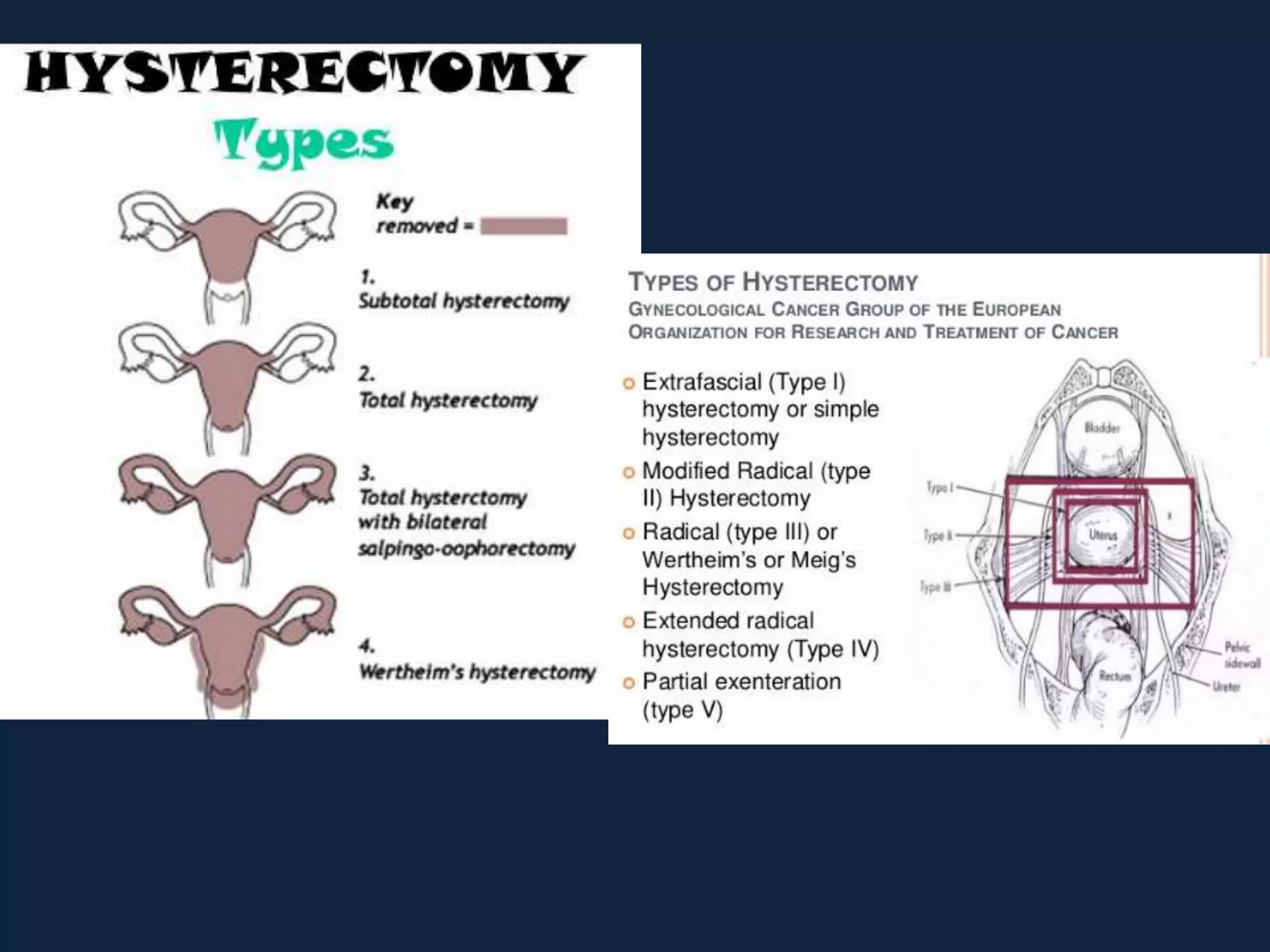
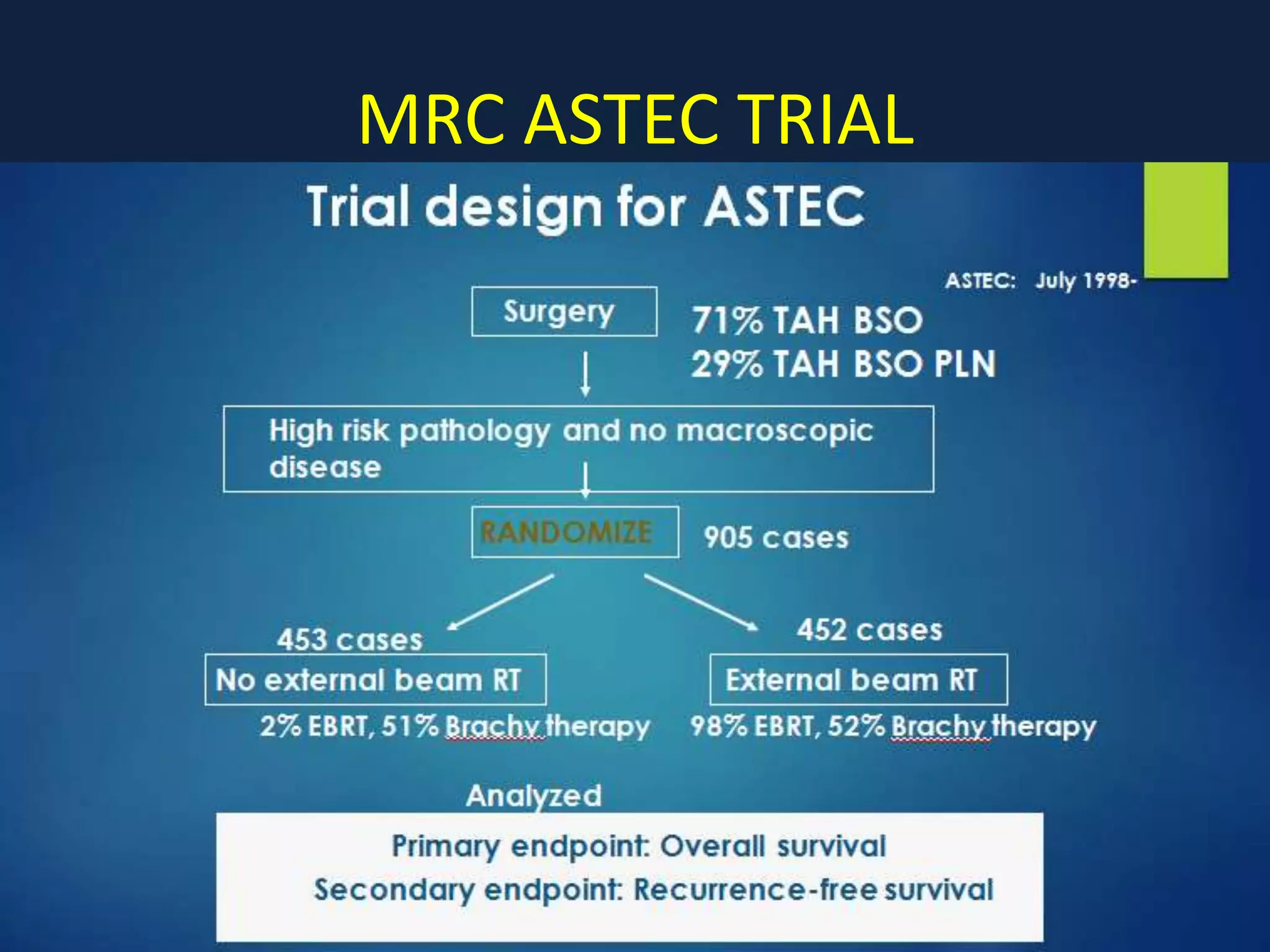
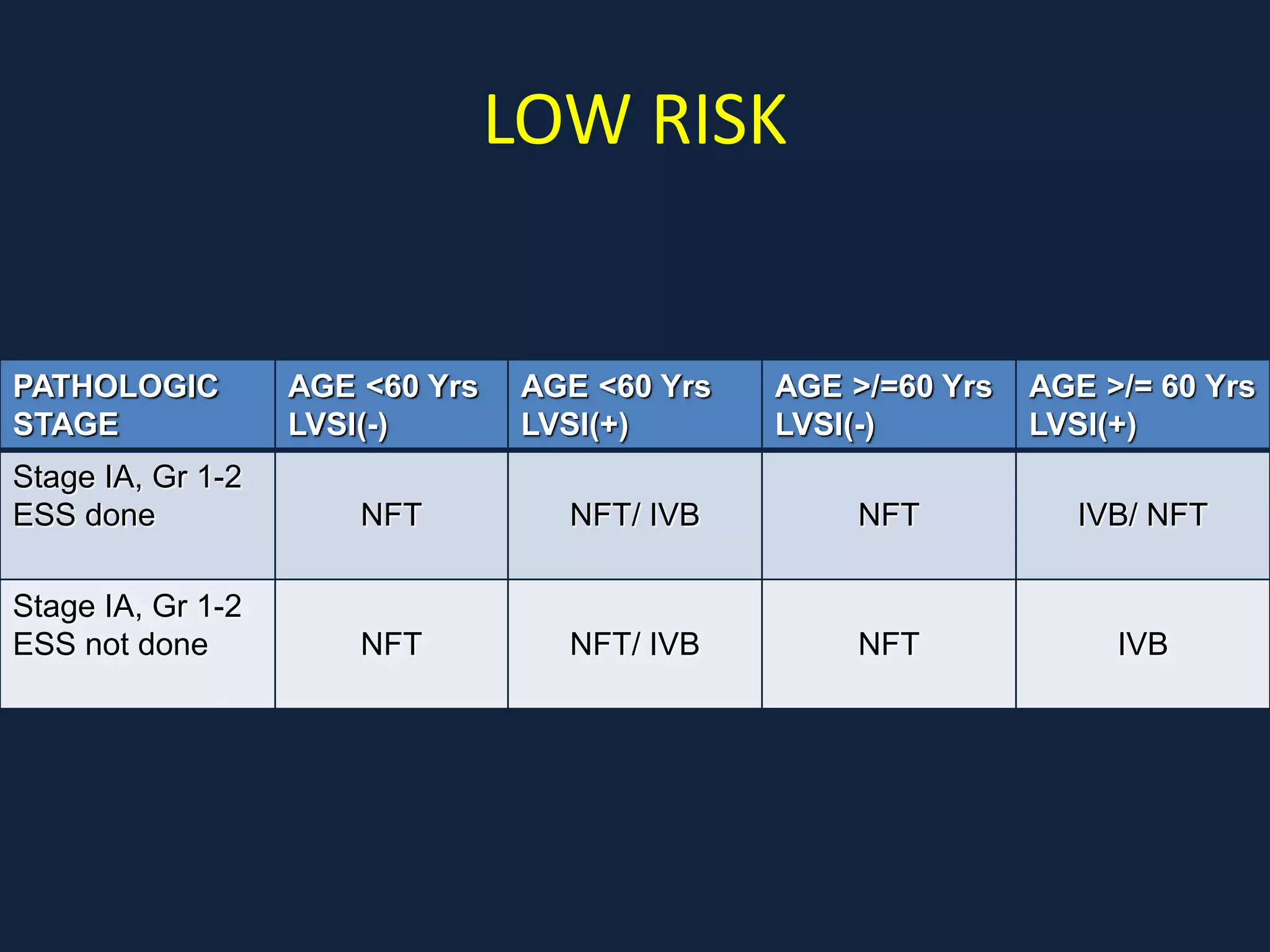
2) Treatment involves surgery (usually a hysterectomy) followed by adjuvant radiation therapy or chemotherapy depending on risk factors and staging. Pelvic radiation therapy techniques have advanced from 4-field box to intensity modulated radiation therapy (IMRT) to reduce toxicity.
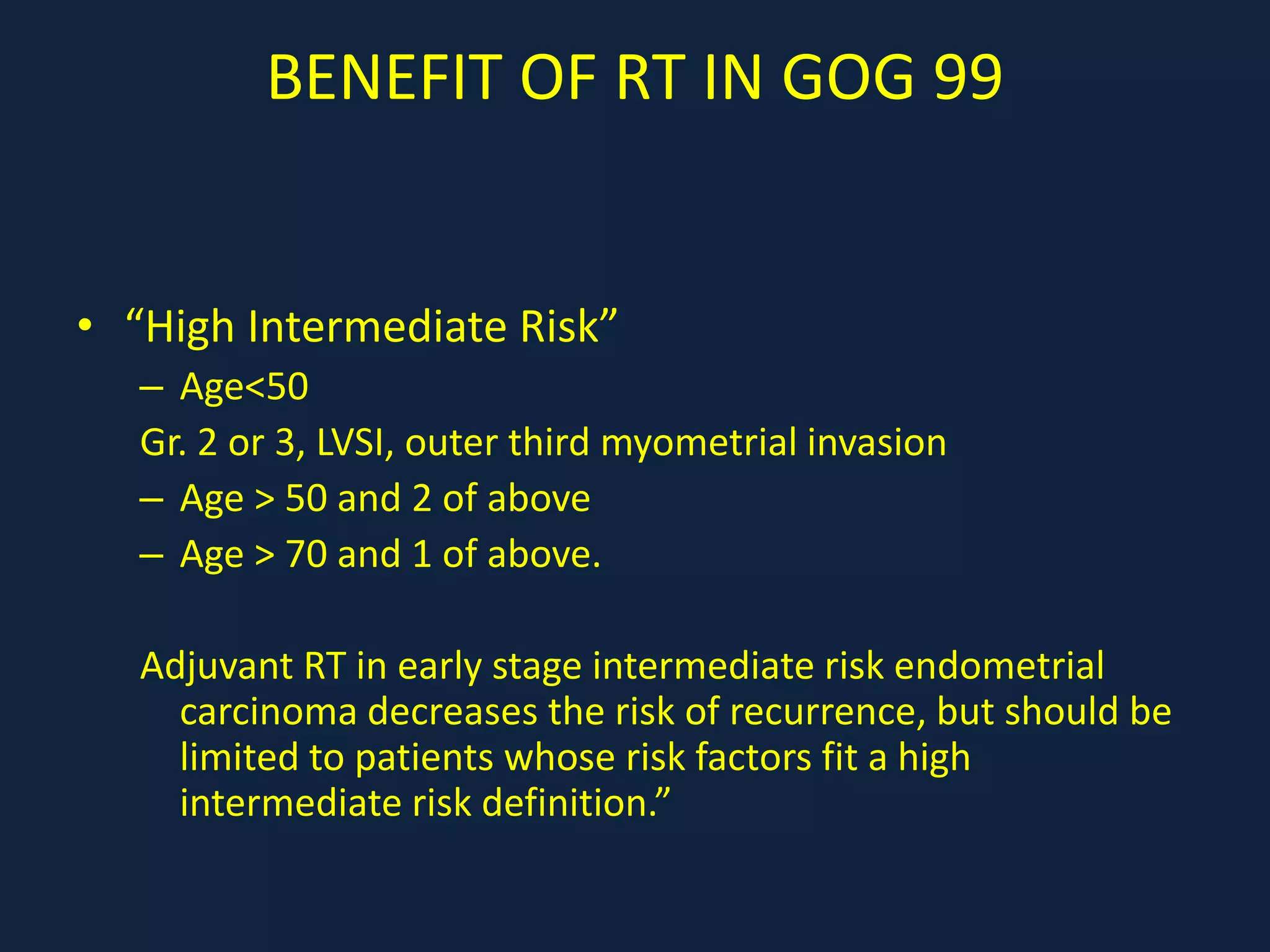
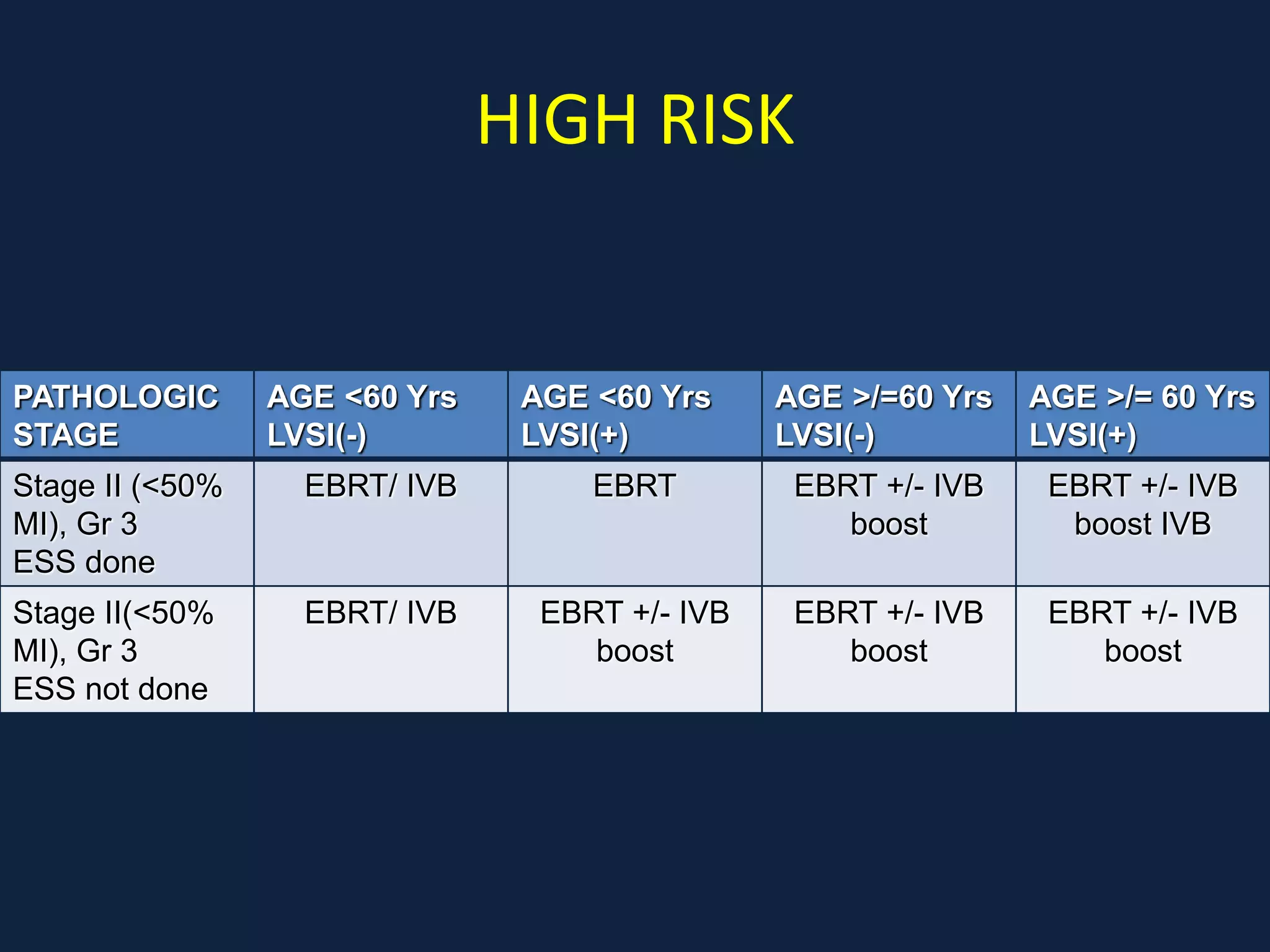
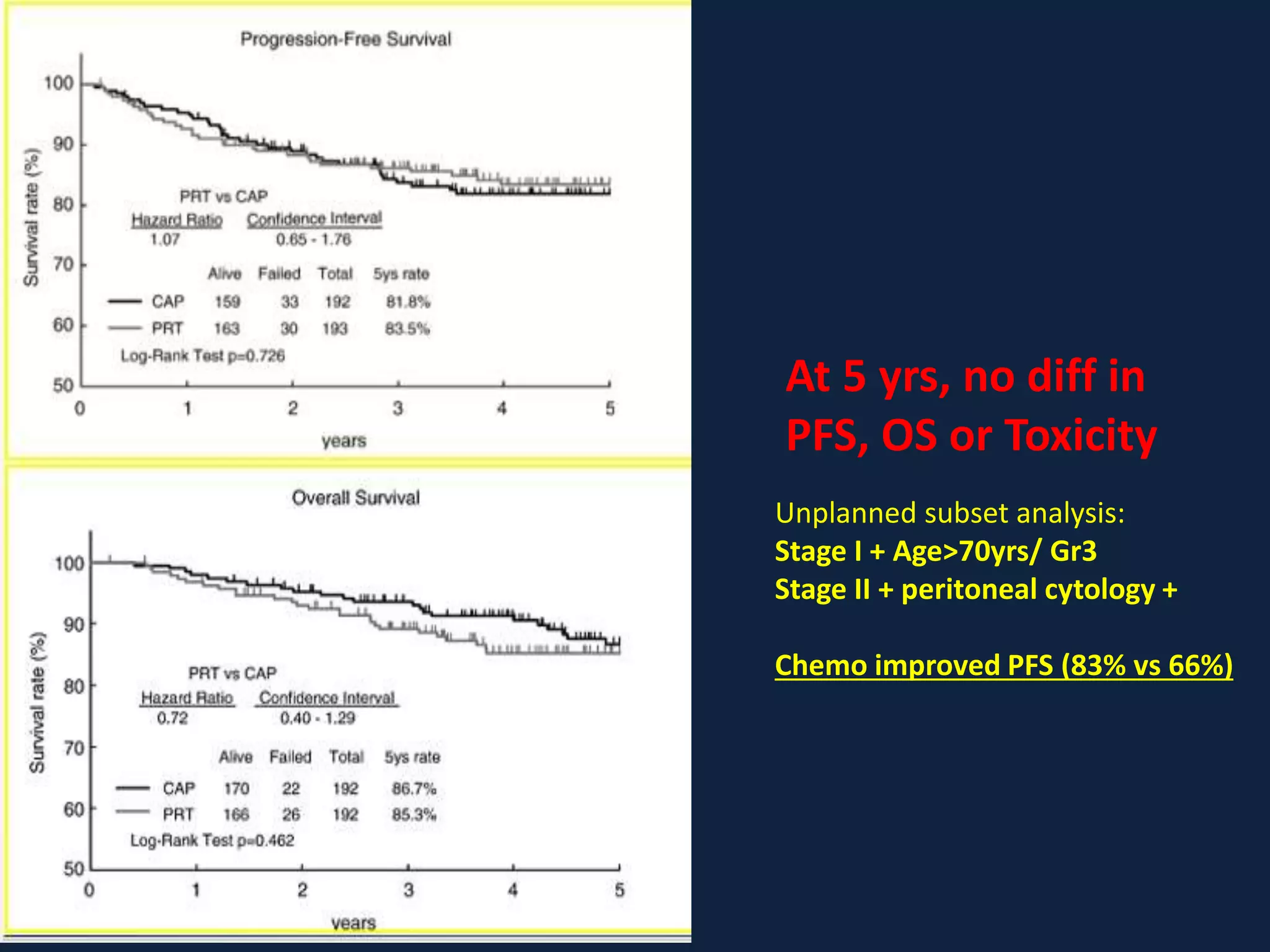
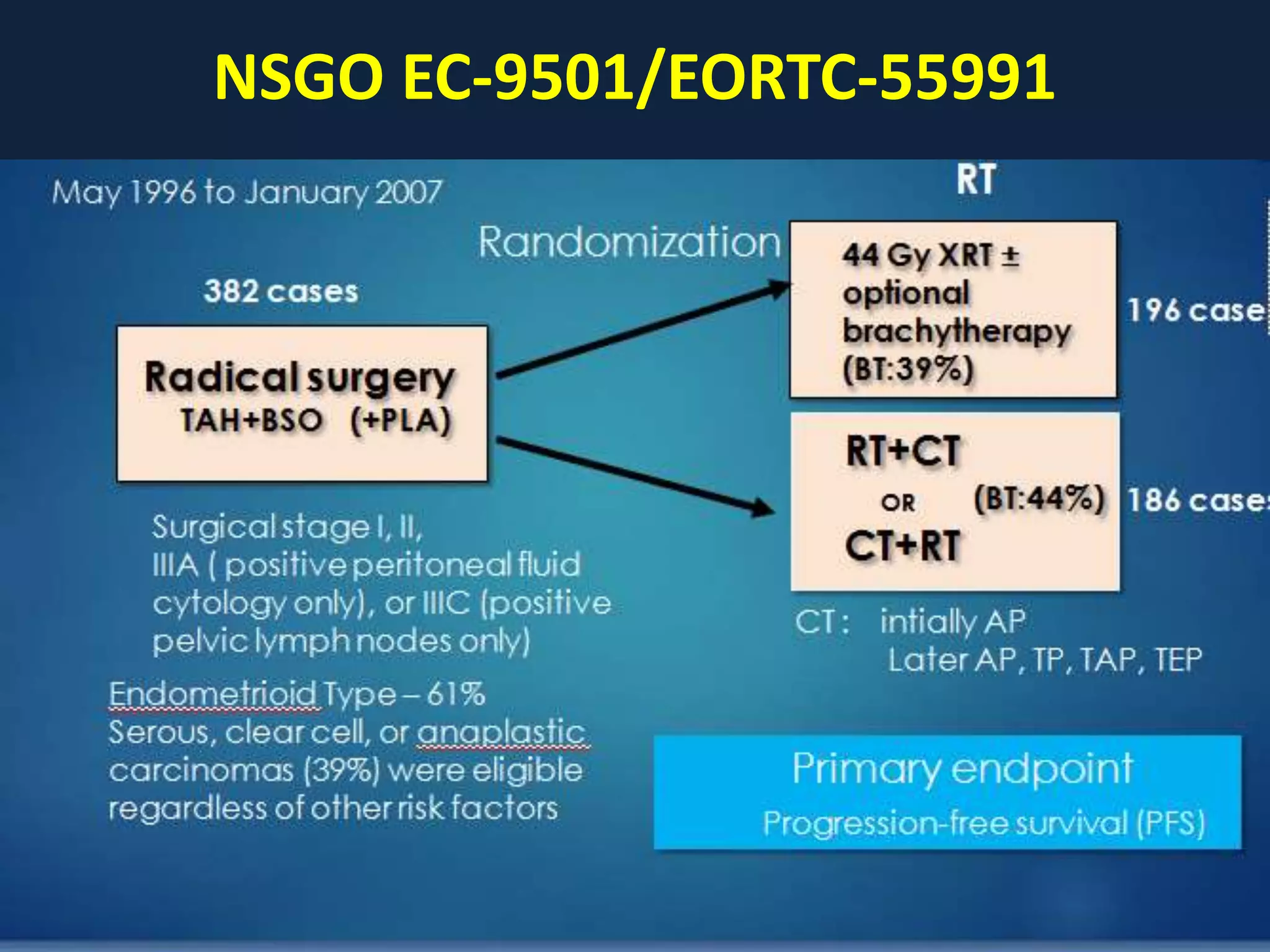
3) Ongoing clinical trials are evaluating the benefits of chemotherapy combined with radiation therapy compared to radiation alone, especially for high risk or advanced stages. Outcomes with current treatments are generally good
































![PORTEC 3
CCRT RT p value
OS 81.8% 76.7% 0.18
FFS 75.5% 68.9% 0.08
Patients with stage III EC had greatest benefit
of CTRT: 5-year FFS 69.3% for CTRT vs 58.0%
for RT p=0.032], and 5-year OS was 78.7 % vs
69.8% (p=0.114).
Adjuvant chemotherapy given during and after pelvic radiotherapy for
treatment of HREC did not significantly improve 5-year FFS and OS, compared
with RT alone. For women with stage III EC FFS was however significantly
improved with CTRT by 11% at 5 years.](https://image.slidesharecdn.com/caendometruim-200520171132/75/Ca-endometruim-44-2048.jpg)