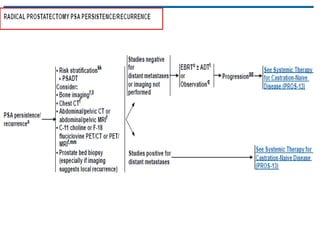
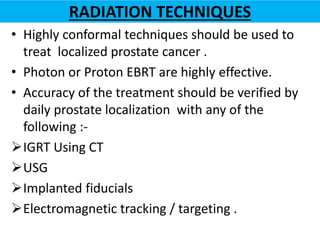
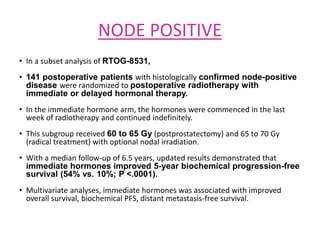
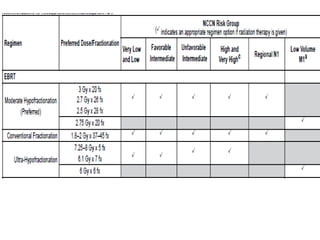
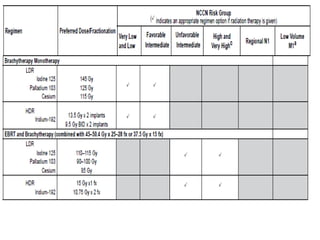
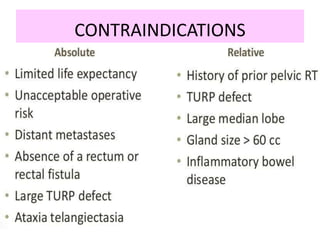
This document summarizes the management of prostate carcinoma. It discusses clinical features, risk stratification, treatment options including active surveillance, radical prostatectomy, radiation techniques, adjuvant and salvage radiation therapy, brachytherapy, and androgen deprivation therapy. Treatment is tailored based on risk factors, tumor characteristics, and patient factors. Image-guided radiation therapy helps ensure accurate targeting of the prostate. Dose escalation and addition of hormones improves outcomes for intermediate- and high-risk disease.