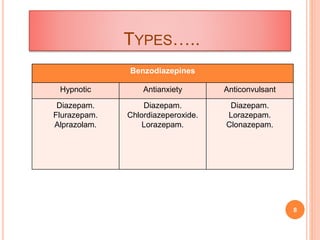
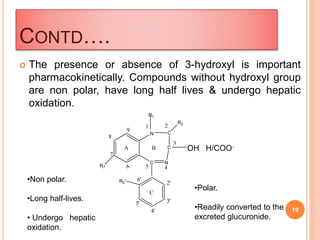
The document discusses benzodiazepines, which are a class of drugs that act as central nervous system depressants. The first benzodiazepine, chlordiazepoxide (Librium), was synthesized in 1955. Benzodiazepines work by enhancing the effects of the neurotransmitter GABA, which has a calming effect on neurons. Some common benzodiazepines include diazepam, alprazolam, and clonazepam, which are used as sedatives, anti-anxiety medications, and anticonvulsants. The molecular structure of benzodiazepines is important for their effects, and modifications to the structure can alter their potency and

![Left: The 1,4-benzodiazepine ring system. Right: 5-phenyl-1H-
benzo[e][1,4]diazepin-2(3H)-one forms the skeleton of many of the most
common benzodiazepine pharmaceuticals, such as diazepam (7-chloro-1-
methyl substituted)
CONTD….
2](https://image.slidesharecdn.com/bzd-230616071418-0087d43c/85/BZD-pptx-2-320.jpg)