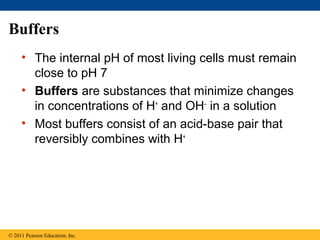
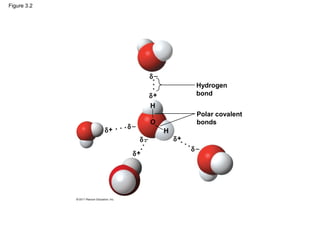
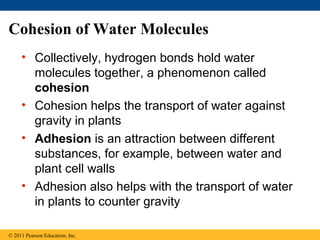
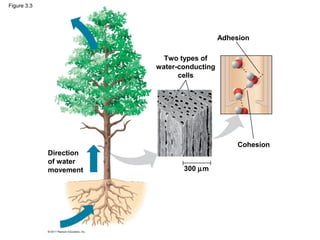
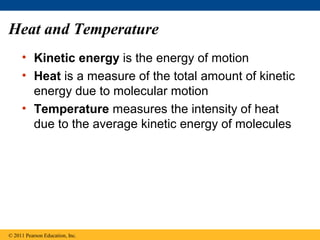
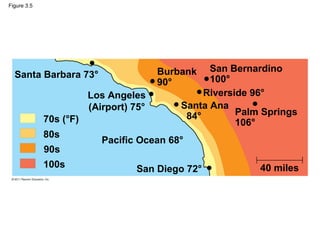
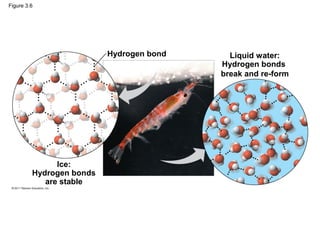
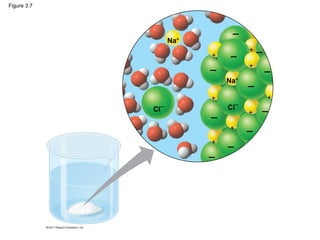
This document provides a summary of key concepts from Chapter 3 of Campbell Biology about water and its importance for life. It discusses how water is the biological medium on Earth, its polarity allows hydrogen bonding, and four properties (cohesion, moderating temperature, expanding when freezing, and being a versatile solvent) contribute to Earth's suitability for life. It also covers how water's properties allow transport in plants, surface tension, heat absorption, specific heat, evaporative cooling, floating of ice, hydrophilic/hydrophobic substances, solute concentration, acid/base chemistry, and the role of buffers.






















![The pH Scale
• In any aqueous solution at 25°C the product of
H+ and OH– is constant and can be written as
[H+][OH–] = 10–14
• The pH of a solution is defined by the negative
logarithm of H+ concentration, written as
pH = –log [H+]
• For a neutral aqueous solution
© 2011 Pearson Education, Inc. [H+] is 10–7 = –(–7) = 7](https://image.slidesharecdn.com/03waterandlife-130311053237-phpapp02/85/03-water-and-life-32-320.jpg)

![Figure 3.10
pH Scale
0
1 Battery acid
Increasingly Acidic
2 Gastric juice, lemon juice
+
H
[H+] > [OH− ]
H+
+
− H Vinegar, wine,
H+ OH 3
+
OH− H H+ cola
H+ H+
Acidic 4 Tomato juice
Beer
solution Black coffee
5
Rainwater
6 Urine
−
OH Saliva
OH− Neutral
H +
H+ OH
−
7 Pure water
OH − OH−
+
[H+] = [OH− ] Human blood, tears
H+ H+ H
8 Seawater
Neutral
Inside of small intestine
solution
Increasingly Basic
9
[H+] < [OH− ]
10
Milk of magnesia
OH−
OH−
OH− H+ OH−
11
OH− OH
− Household ammonia
H + OH−
12
Basic
solution Household
13 bleach
Oven cleaner
14](https://image.slidesharecdn.com/03waterandlife-130311053237-phpapp02/85/03-water-and-life-34-320.jpg)