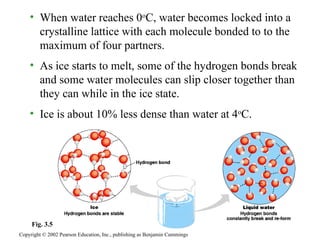
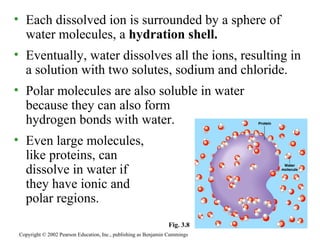
The document summarizes several key properties of water that make it essential for life. It discusses how (1) the polarity and hydrogen bonding of water molecules allows it to act as a solvent for polar substances and moderate temperatures on Earth. It notes that (2) oceans and lakes don't freeze solid because ice floats, and (3) water is the universal solvent that has allowed for the development of life.