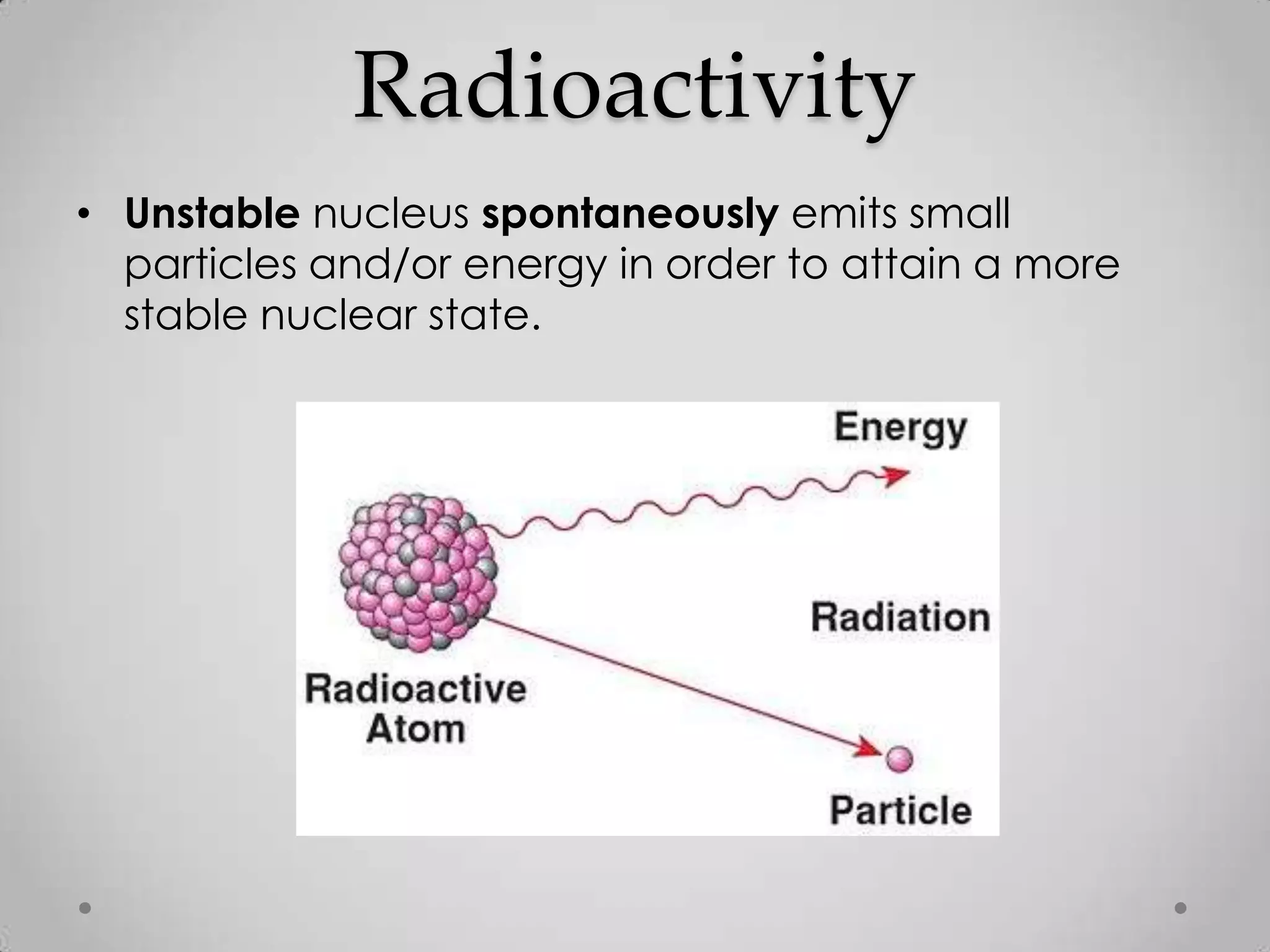
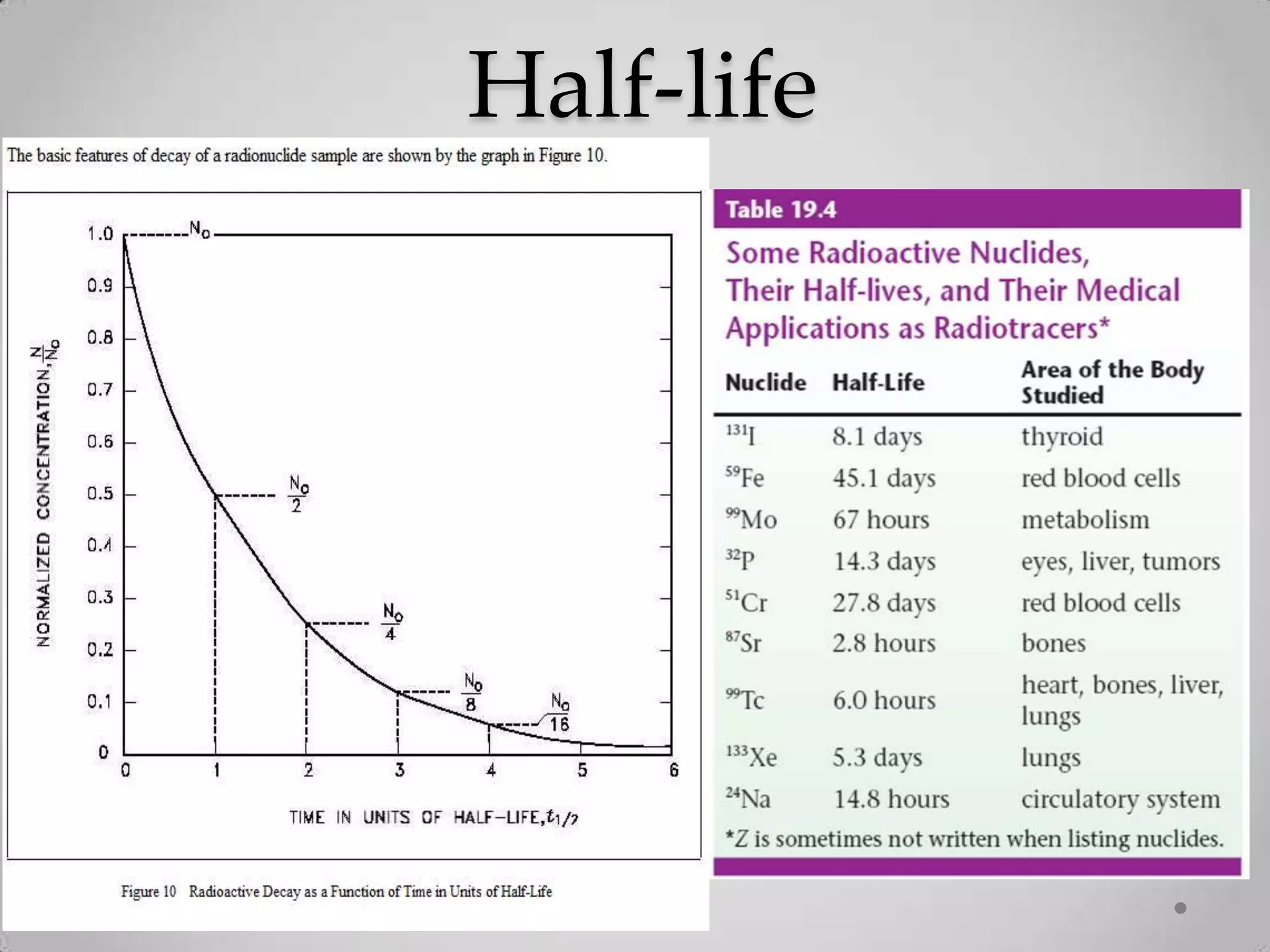
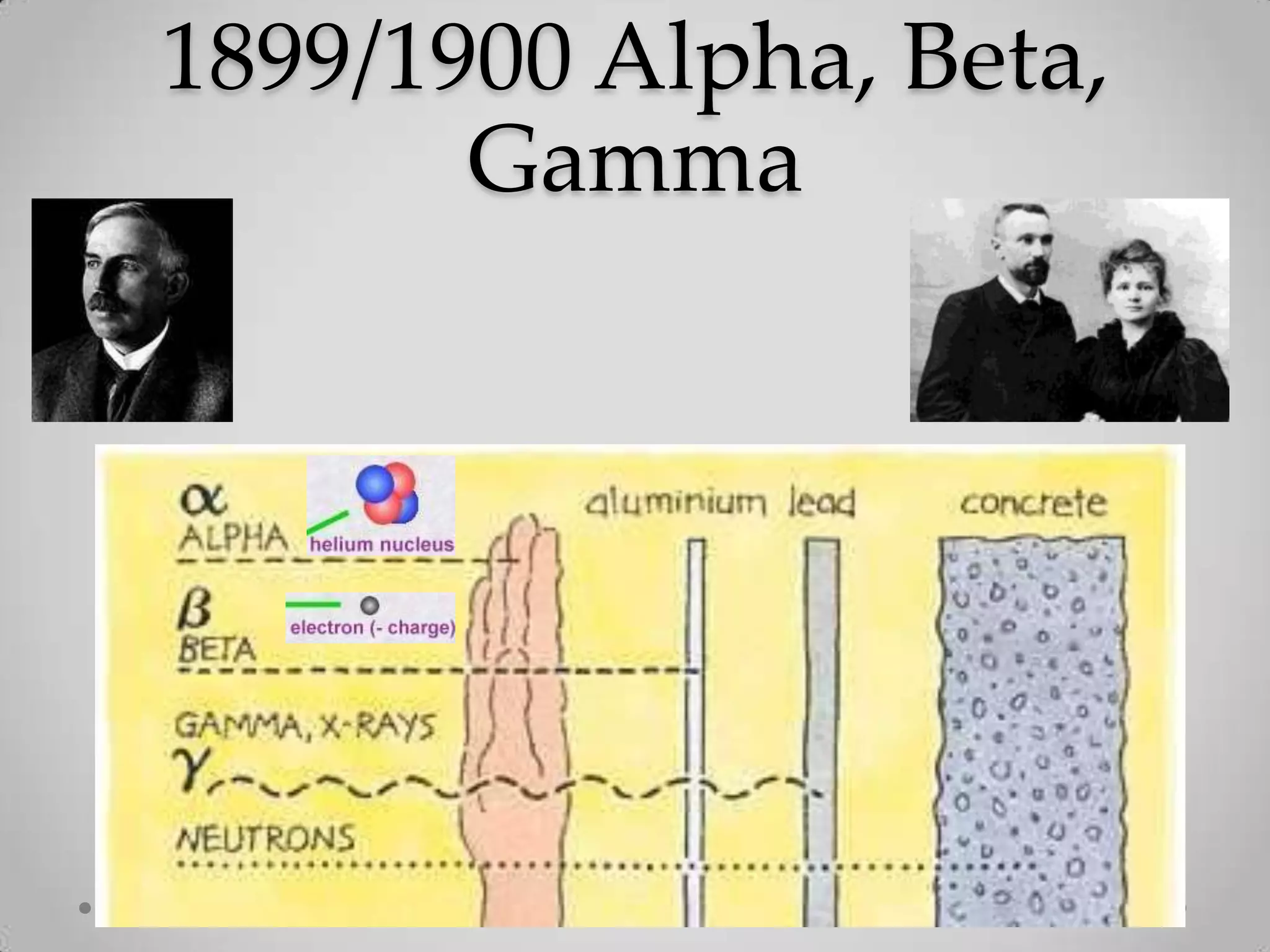
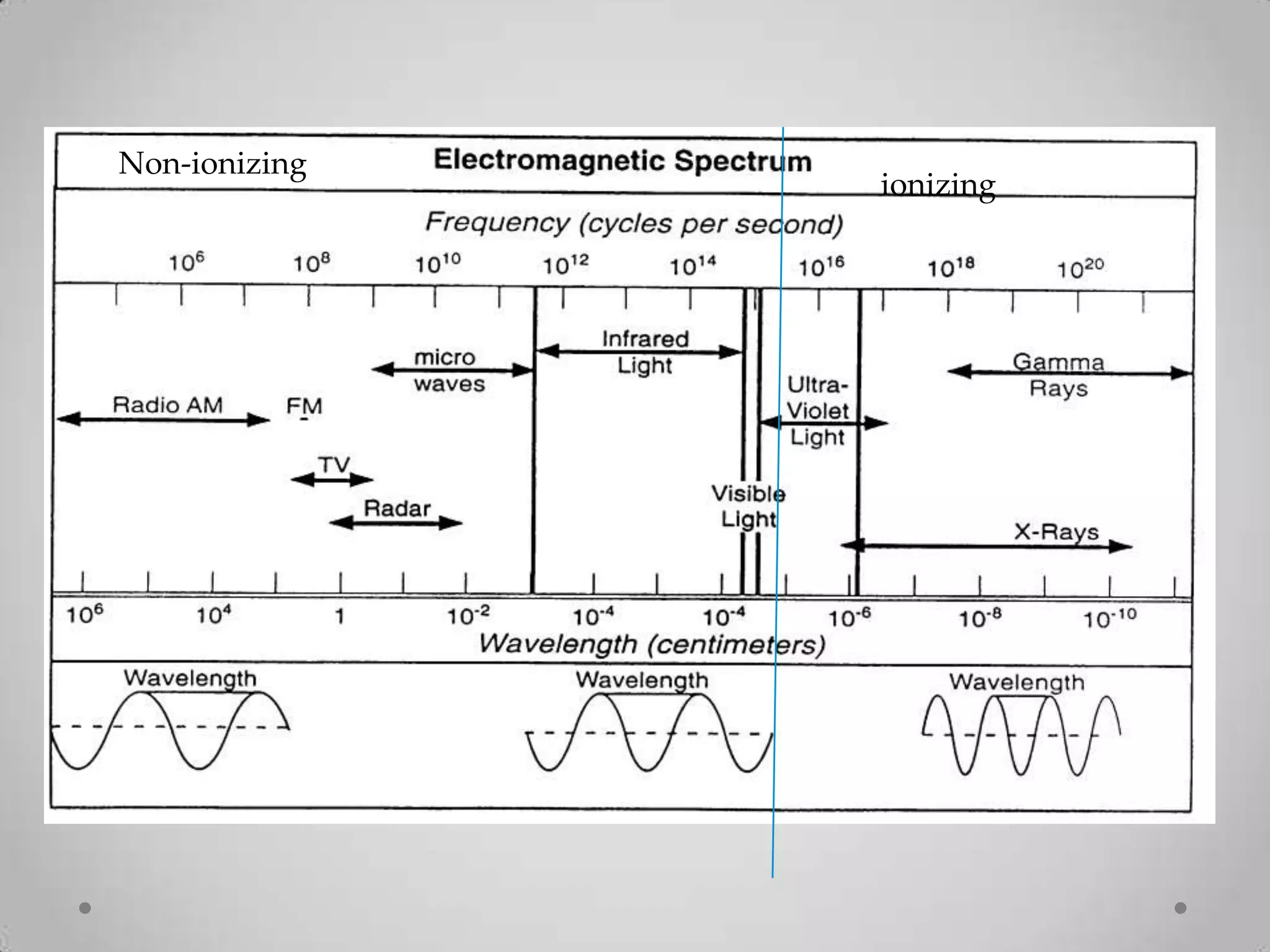
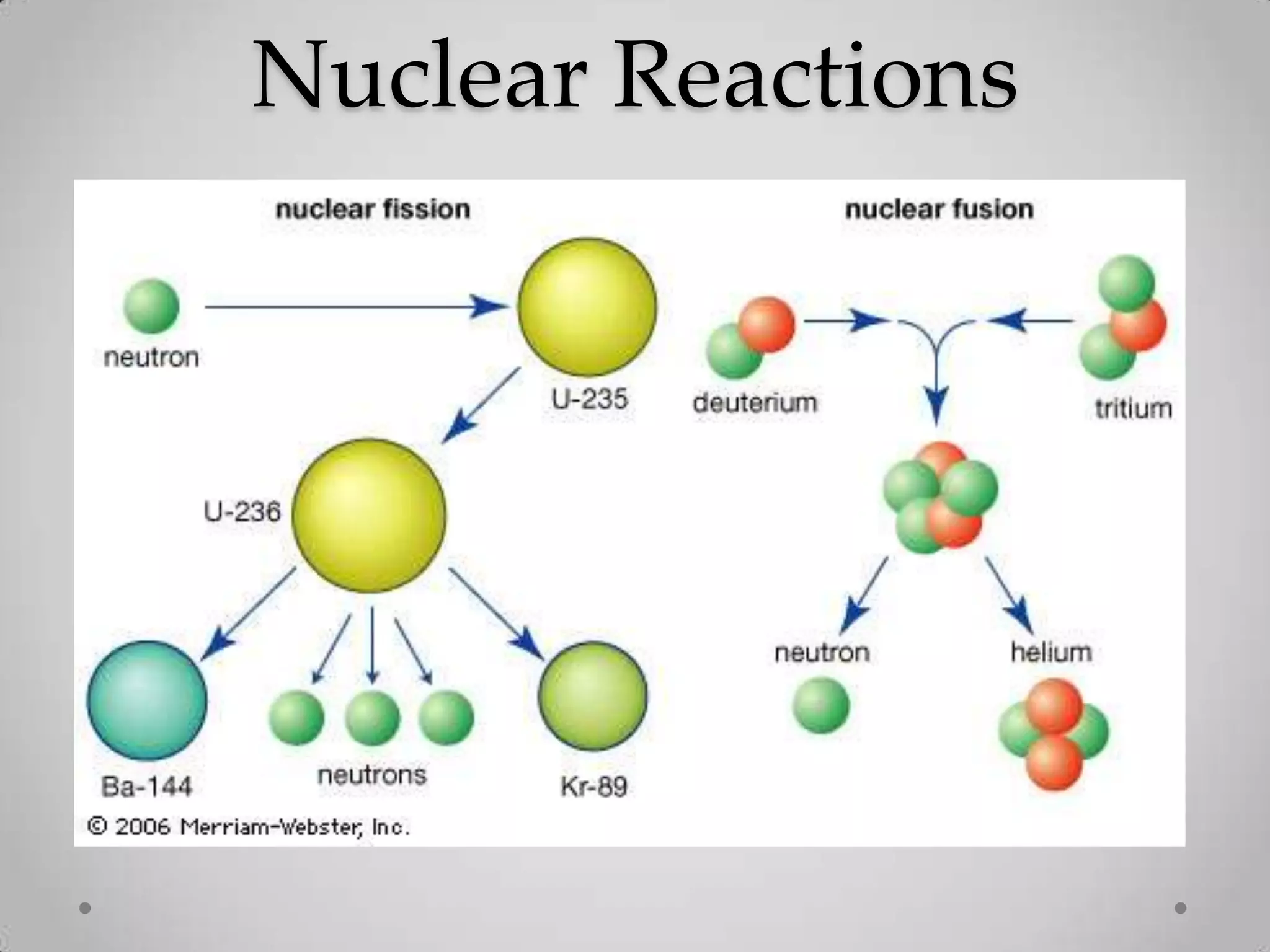
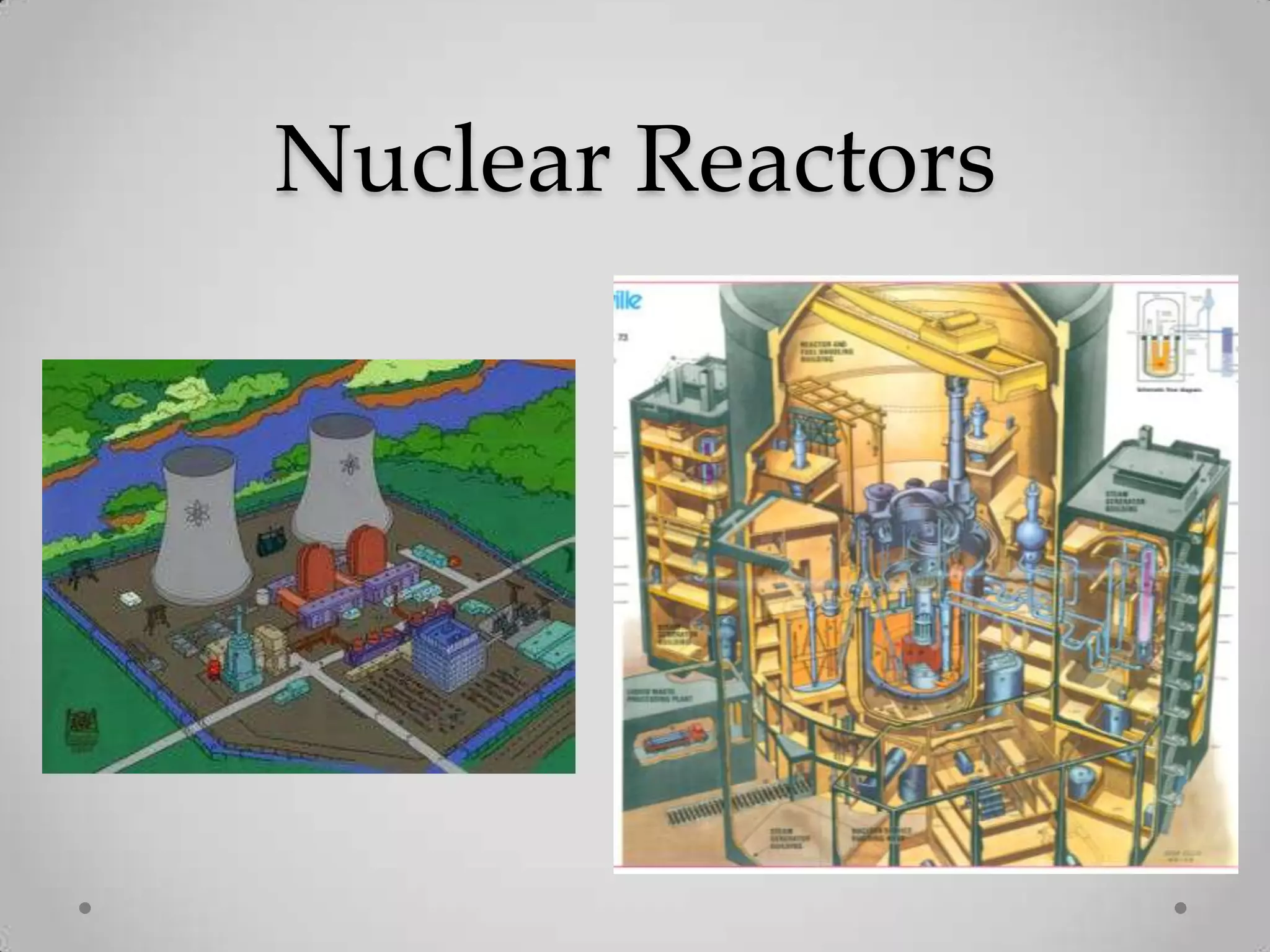
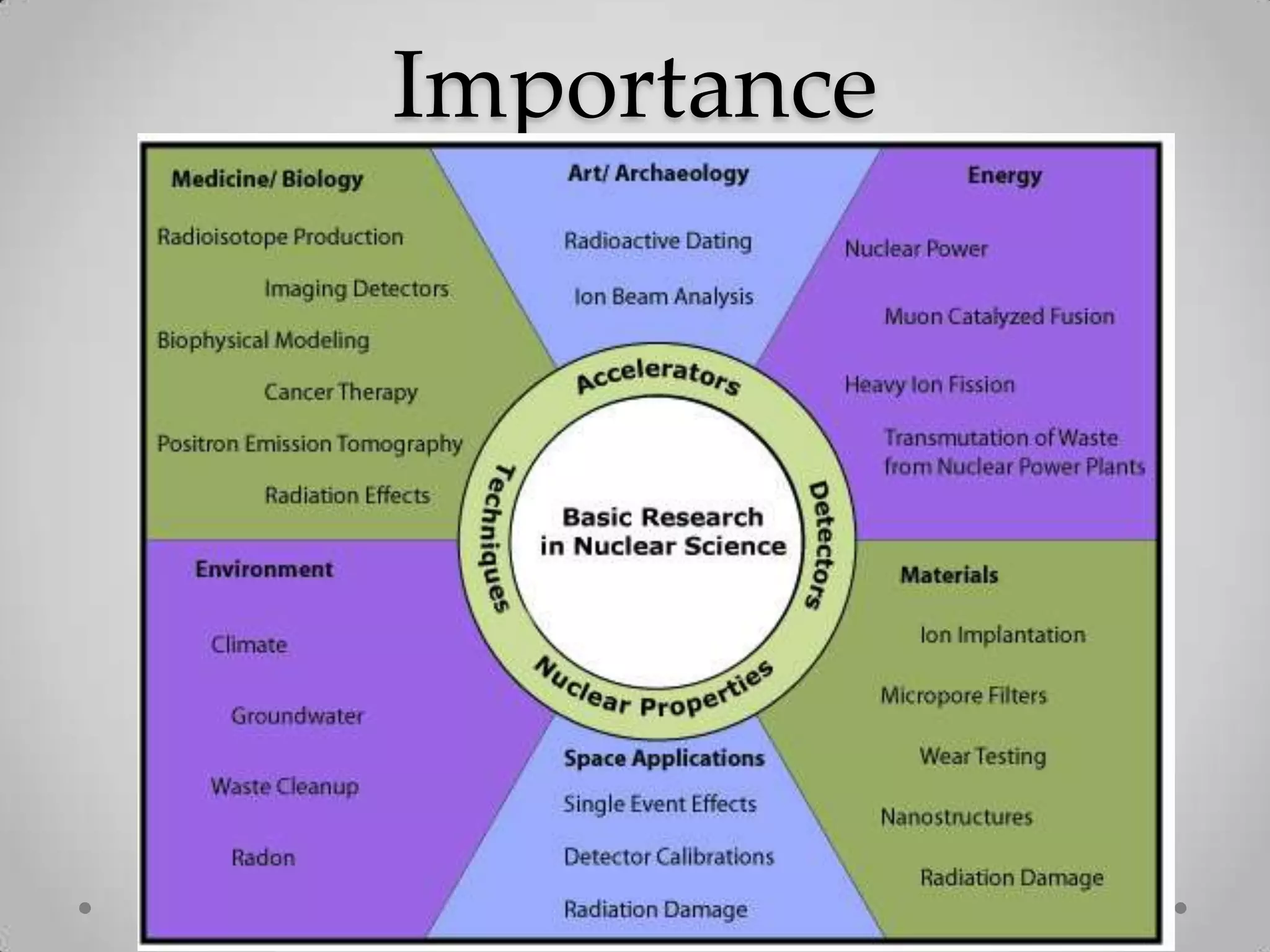
Radioactivity is the spontaneous emission of particles or energy from unstable atomic nuclei to become more stable. It was discovered in 1896 by Henri Becquerel when studying uranium salts that emitted rays and could burn skin. Later, Marie and Pierre Curie isolated the radioactive elements radium and polonium, and defined their property of half-life. There are three main types of radioactive emissions - alpha, beta, and gamma rays - which were categorized by their ability to ionize atoms as either ionizing or non-ionizing radiation.