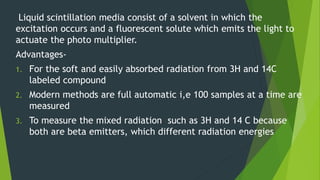
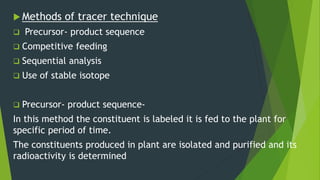
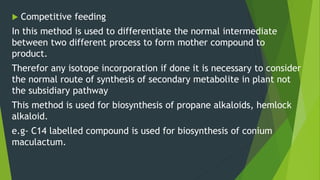
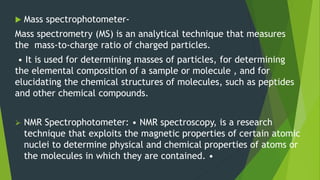
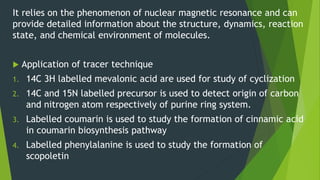
This document discusses techniques used to study biosynthetic pathways in plants. It describes the shikimic acid pathway and mevalonate pathway, which are important for producing aromatic compounds and terpenes respectively. It also summarizes several techniques used in biogenic studies including the use of isolated organs, grafting, mutant strains, tracer techniques, and enzymatic studies. Tracer techniques involve using radioactive isotopes or stable isotopes to trace metabolic pathways and locate compounds. Proper labeling and introduction of tracers is required.














![ Labelled compound can be prepared by use of two type of isotope
1. Radioactive isotopes: • [e.g. 1H, 14C, 24Na, 42K, 35P, 131I decay
with emission of radiation]
• For biological investigation – carbon & hydrogen.
• For metabolic studies – S, P, and alkali and alkaline earth metals are
used.
• For studies on protein, alkaloids, and amino acid – labelled nitrogen
atom give more specific information.
• 3H compound is commercially available.](https://image.slidesharecdn.com/unitibiosyntheticpathways-230510052709-f8002f46/85/Biosynthetic-pathways-15-320.jpg)
![2. Stable isotope
• [e.g. 2H, 13C, 15N, 18O]
• Used for labeling compounds as possible intermediates in biosynthetic
pathways.
• Usual method of detection are: – Mass spectroscopy [15N, 18O] • NMR
spectroscopy [2H, 13C]
2. Introduction of labelled compounds
• Precautions: • •
1. The precursor should react at necessary site of synthesis in plant.
2. Plant at the experiment time should synthesize the compound under
investigation
3. The dose given is for short period.](https://image.slidesharecdn.com/unitibiosyntheticpathways-230510052709-f8002f46/85/Biosynthetic-pathways-16-320.jpg)