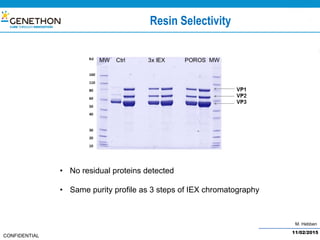
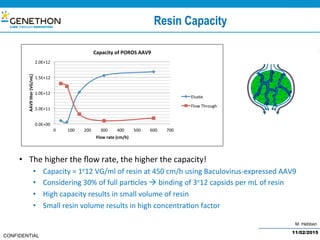
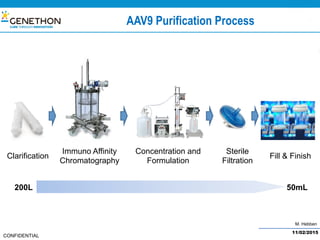
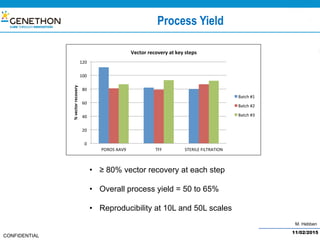
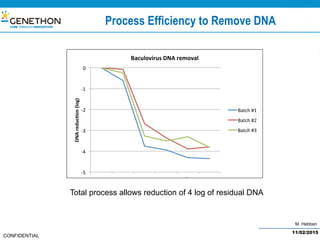
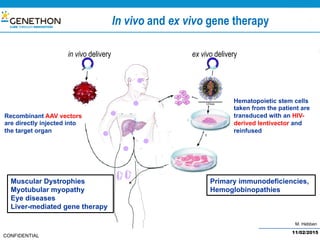
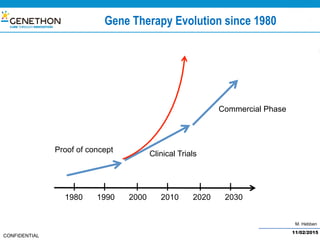
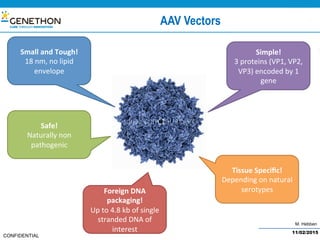
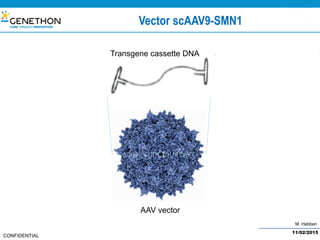
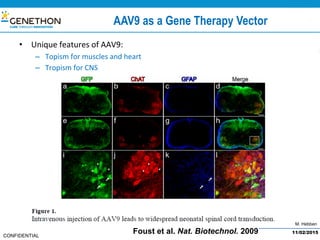
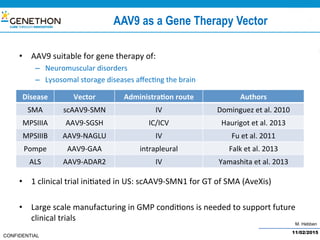
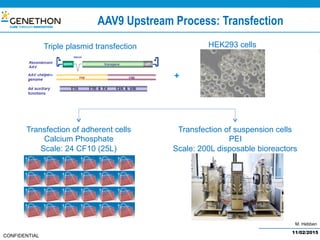
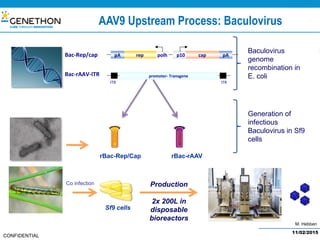
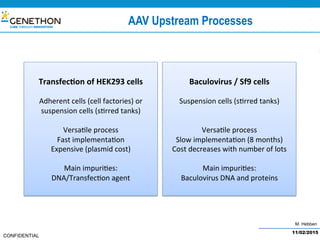
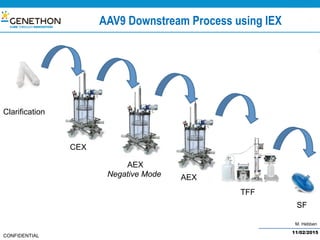
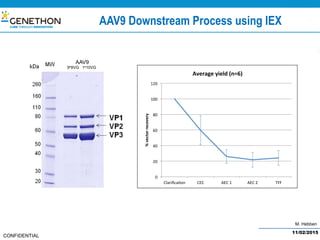
The document discusses the development and manufacturing challenges of AAV9 gene therapy vectors, highlighting the history and evolution of gene therapy from the 1980s to the present. It details GeneThon's operations, including its GMP manufacturing capabilities and the effectiveness of AAV9 in treating various diseases, as well as the purification processes necessary for large-scale production. The conclusion emphasizes that manufacturing AAV9 vectors at a large scale is feasible with a high yield process, suitable for clinical applications.


![M. Hebben
CONFIDENTIAL
POROS®CaptureSelect™ AAV9
• Affinity resin developed by Thermo Fisher Scien@fic
• Ligand based on a single-domain [VHH] an@body fragment
• VHH affinity ligands are produced in yeast (S. cerevisiae) in an
animal origin free produc@on process
– ISO9001 cer@fied manufacturing facility (Netherlands)
12-15kDa
• Small size:12-15 kDa
fragment: ~1/10th mAb
• Tunable specificity/affinity
CaptureSelect™ technology
Caution: For manufacturing, processing, or repacking.](https://image.slidesharecdn.com/bils2015mhebben-160418133722/85/BILS-2015-Genethon-M-Hebben-17-320.jpg)