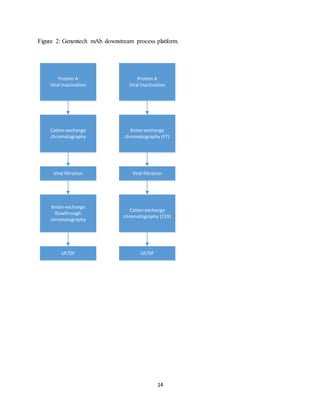
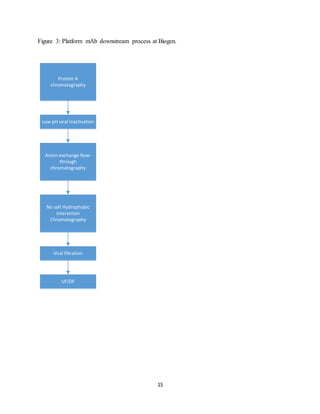
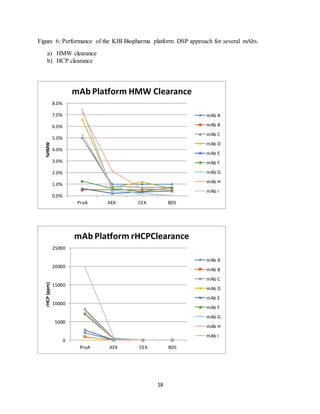
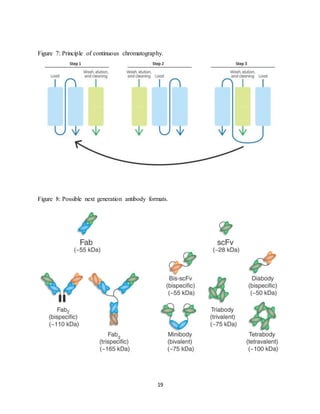
This document discusses the evolution of downstream process platforms for monoclonal antibodies (mAbs), emphasizing their role in accelerating development and production efficiencies. It highlights various current and emerging technologies, including continuous chromatography and multimodal chromatography, that enhance the purification and scalability of mAb manufacturing processes. The importance of adapting these platforms to meet the specific requirements of different mAb constructs and regulatory standards is also addressed.